By Bill Griffin, Special Contributor to New Frontier Data
In 2019, France announced plans to commence a medicinal cannabis pilot, which is now due to start in September 2020. According to New Frontier Data, the country's cannabis consumer market is worth an estimated € 9,437,336,621.
The pilot will run for two years with the initial six months will serve to identify the 3000 patients who will most benefit from the programme. The second six months will focus on recruiting hospitals, private clinics and multidisciplinary centres where the specialized doctors, physicians and clinicians will be located. All participating medical professionals will be required to go through an extensive online training programme. One these steps are completed, the pilot is expected to be fully operational, and the first prescriptions for medicinal cannabis are expected to start in January 2021.
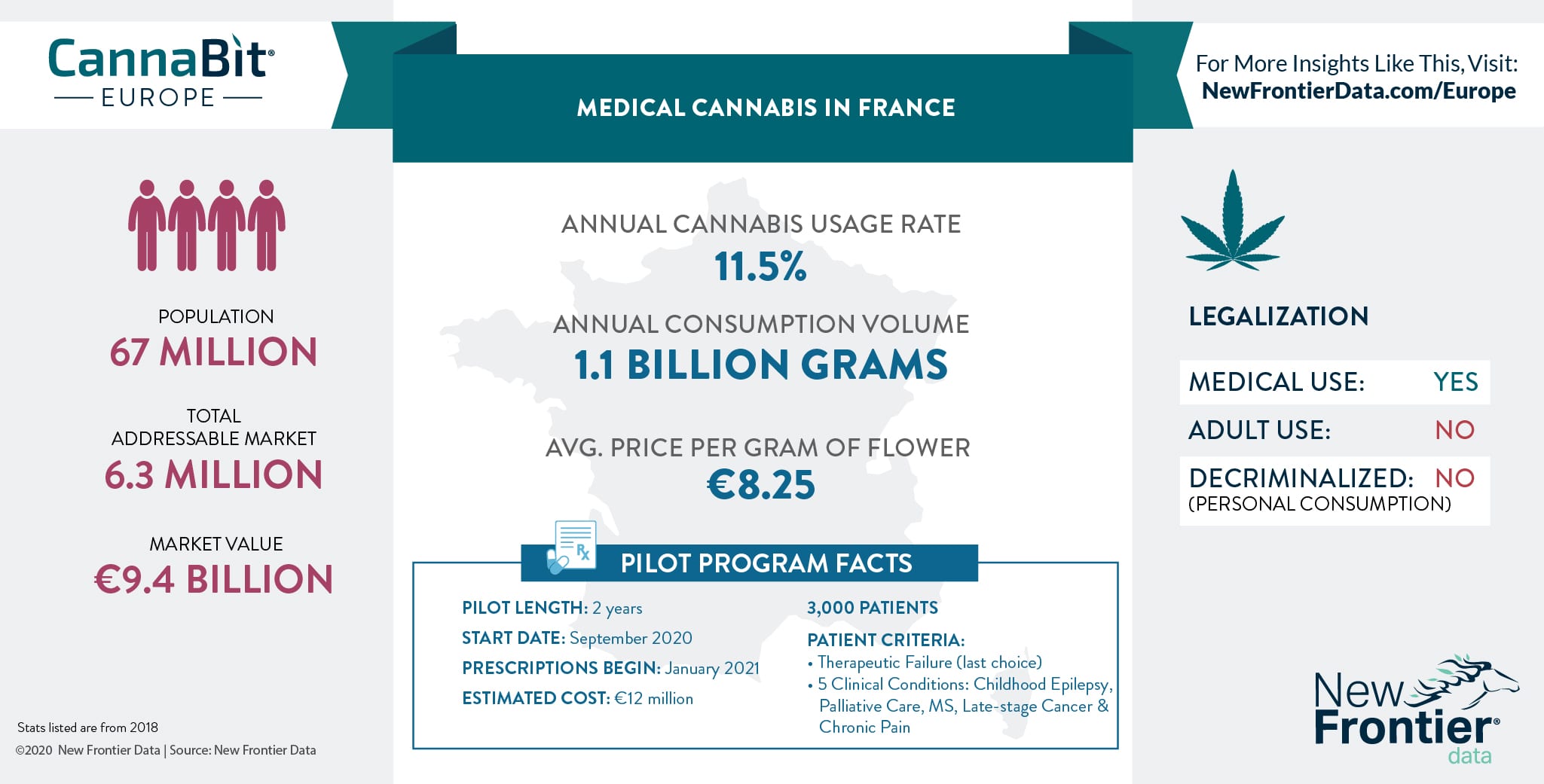 There are two criteria in which the pilot will select the first patients. The first, is a therapeutic failure where the patient has exhausted all of his/her options and is unable to find relief from a chronic condition. The second, is a list of five therapeutic indications including: epilepsy in children, palliative care, multiple sclerosis, late stage cancer (to offset side effects of chemotherapy), and chronic pain where other solutions have proven ineffective.
There are two criteria in which the pilot will select the first patients. The first, is a therapeutic failure where the patient has exhausted all of his/her options and is unable to find relief from a chronic condition. The second, is a list of five therapeutic indications including: epilepsy in children, palliative care, multiple sclerosis, late stage cancer (to offset side effects of chemotherapy), and chronic pain where other solutions have proven ineffective.
"The initial patient selection criteria is quite restrictive," says Béchir Saket Vice-President of L630, a drug policy reform network of experts from civil society who are advising the government on how to best approach the pilot. "This is a shame, as ultimately only 3000 people will benefit from phase one of the pilot. In reality, at least 300,000 patients could be helped by such a programme. Should the results of the initial pilot be positive, it's hoped that it will be extended to more doctors and patients in the future." L630 represents ACT Collective, an umbrella organization of French patient groups.
As part of the pilot, patients will gain access to an estimated one gram per day of pharmaceutical-grade cannabis flower (or the equivalent). In total, this is expected to cost an estimated €12 million. This cost will be borne by the companies supplying the material, who have not yet been publicly identified, however, negotiations are reported to be in the advanced stage. It is worth noting that this €12 million estimate is the final sale price and does not represent the actual cost to these companies.
For the duration of the pilot, the companies providing cannabis products will be able to import. However, it is clear that should the pilot transform into a fully-regulated medicinal cannabis programme, the government will expect the product to be cultivated and processed in France.
Once the two-year pilot is complete, a study by the French government will be sent to Parliament who will then decide whether to continue with the programme and in what format.
Currently open for debate is whether, and how, costs for medicinal cannabis products will be reimbursed to patients. Traditional pharmaceutical companies in France are notably strongly opposed to this. Medical cost reimbursement in France is already a complicated affair, and many medicines have gone through extensive trials with major pharmaceutical investments do not qualify. These companies are aggrieved that a cannabis company could simply set up a grow facility, cannibalize on their profit margins, and then receive full reimbursement from the government. One should expect intense lobbying efforts concerning this point.
Debates are also already underway on the regulatory framework post-completion of the pilot programme. A government commission on hemp and cannabis regulations has been formed, comprised of a working group of 33 high profile French MPs from across the political spectrum. This is the first of such a commission in France, and it offers a chance for a real debate on the future of French medicinal cannabis and regulations that will ultimately effect millions of patients.
The post Delayed French Medical Cannabis Pilot Programme Planned for Q3 Launch appeared first on New Frontier Data.
The preceding article is from one of our external contributors. It does not represent the opinion of Benzinga and has not been edited.
Image Sourced from Pixabay
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
Cannabis is evolving – don’t get left behind!
Curious about what’s next for the industry and how to leverage California’s unique market?
Join top executives, policymakers, and investors at the Benzinga Cannabis Market Spotlight in Anaheim, CA, at the House of Blues on November 12. Dive deep into the latest strategies, investment trends, and brand insights that are shaping the future of cannabis!
Get your tickets now to secure your spot and avoid last-minute price hikes.