Zinger Key Points
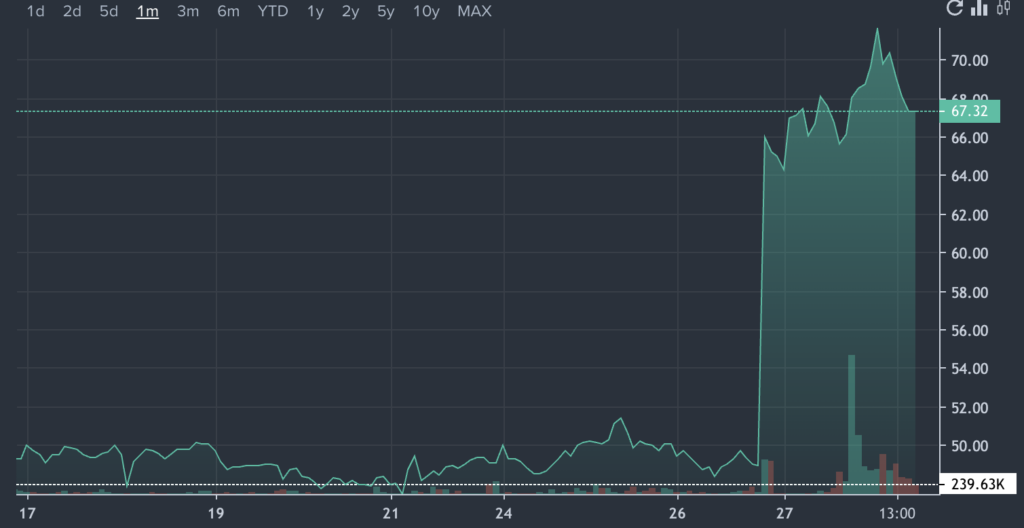
- Soleno Therapeutics shares jumped 37.2% after securing FDA approval for Vykat XR, its first commercial drug for treating hyperphagia.
- The approval was based on Phase 3 trial data showing significant effectiveness, with the drug expected to launch in the U.S. by April 2025.
- Feel unsure about the market’s next move? Copy trade alerts from Matt Maley—a Wall Street veteran who consistently finds profits in volatile markets. Claim your 7-day free trial now.
Soleno Therapeutics, Inc. SLNO shares are trading higher Thursday after the company secured FDA approval for its first commercial drug, Vykat XR (diazoxide choline) extended-release tablets.
What To Know: The drug is approved to treat hyperphagia in adults and children aged 4 years and older with Prader-Willi syndrome (PWS), a rare genetic disorder that causes insatiable hunger and affects metabolism, body function and behavior.
The approval was based on data from a Phase 3 clinical trial, where patients who switched to a placebo experienced a significant worsening of hyperphagia compared to those who remained on Vykat XR. The drug has demonstrated a well-established safety profile with over four years of data across multiple studies.
Soleno expects to launch Vykat XR in the U.S. by April 2025. The company has been preparing for the commercial rollout with investments in disease education, data analytics, and infrastructure. The FDA had extended its review of the drug in November, with the final decision arriving on March 27, 2025.
SLNO Price Action: Soleno shares were up 34.9% at $66.04 at the time of writing, according to Benzinga Pro.
Read Next:
Image via Soleno Life.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.