Zinger Key Points
- Arcellx replaces Sarepta Therapeutics in Needham Conviction List.
- In 2023, Arcellx stock appreciated around 75% versus the S&P 500, up around 24%
- China’s new tariffs just reignited the same market patterns that led to triple- and quadruple-digit wins for Matt Maley. Get the next trade alert free.
Needham analyst forecasts a positive outlook for Arcellx Inc ACLX in 2024, citing strong potential stemming from the anticipated pivotal interim readout from the iMMagine-1 study, expected in the second half of 2024.
This development is poised to bolster filing prospects for the company in 2025. CART-ddBCMA (anitocabtagene autoleucel or anito-cel), the flagship product of Arcellx, displays promising potential for value creation, particularly upon disclosing its clinical design for deployment in earlier line settings.
Needham analyst reiterates a Buy rating on ACLX, raises the price target to $71 from $65, and adds ACLX to the Needham Conviction List, replacing Sarepta Therapeutics Inc SRPT.
According to the analyst, Anito-cel showcases comparable or potentially superior efficacy to the market leader, Johnson & Johnson JNJ/Legend Biotech LEGN Carvykti, while demonstrating a notably improved safety profile.
Notably, in the Phase I study conducted in 2023, Anito-cel displayed an upfront Overall Response Rate and durability on par with Carvykti, even outperforming it in treating patients with extramedullary disease.
The overall sentiment from the analysis anticipates a favorable trajectory for Arcellx in 2024, emphasizing the company’s relatively de-risked asset status.
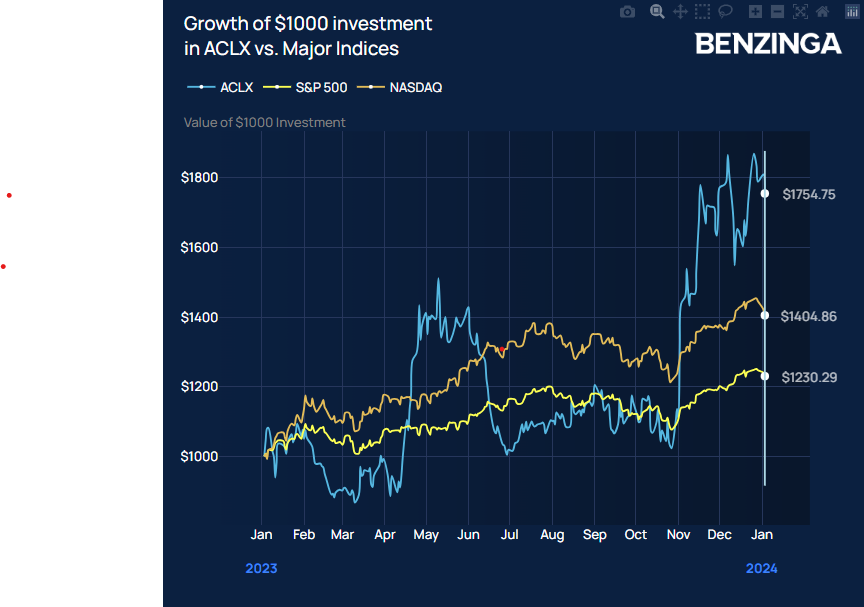
In 2023, Arcellx stock appreciated around 75% versus the S&P 500, up around 24%.
Needham notes ACLX has room to grow, assuming anito-cel can capture a significant portion of the ~$20 billion market opportunity across multiple myeloma.
Price Action: ACLX shares are up 2.70% at $55.92 on the last check Thursday.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.