Aveo Pharmaceuticals Inc. AVEO shares are trading higher Thursday after the company received FDA approval for Fotivda for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma on Wednesday evening.
After a higher open Thursday morning, the stock was down 15.58% at $12.90 at last check. 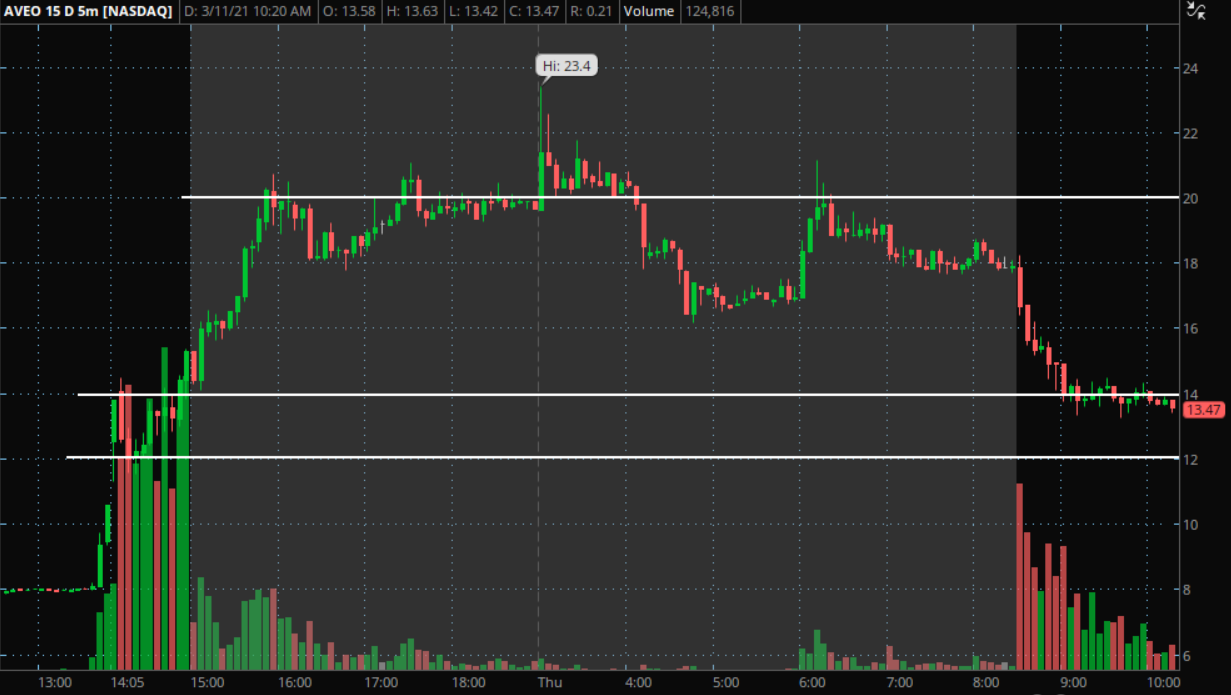
Aveo Technical Levels To Watch: The short-term, 5-minute chart above shows that the price started moving after the FDA approval announcement before the close Wednesday. The price continued to rise throughout extended hours before selling off during Thursday’s trading session.
At the start of Wednesday’s run, the price found some resistance initially near $14. It then fell to $12 and consolidated before the stock really started moving in after-hours trading. These are potentially key areas to watch.
The 5-minute chart seems to be showing some consolidation near the $14 area for a potential bounce on Thursday. If this bounce did not occur and support were to break, the next potential level for a bounce may be near the $12 area, as this is where the stock was able to find support before.
The stock may run into a resistance level somewhere near $20, as this is an area the price has struggled to cross before.

The daily chart above shows the price has had a very fast increase following the FDA approval. Previous history on the daily chart shows the stock has had trouble getting over the $10 level. This level may be a potential level of support in the future.
The FDA approval caused new highs on the daily chart. The only potential resistance shown from previous chart history is the highs the stock made on the chart near $18.
Aveo is trading with a market cap of $335 million and a public float of 24.78 million shares.
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Comments
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.