Novavax NVAX submitted an application to the FDA, seeking to amend its Emergency Use Authorization (EUA) and update its protein-based COVID-19 vaccine for the upcoming fall season.
While this updated vaccine has been formulated to target the JN.1 variant, management claims that the vaccine has also been shown to be effective against the current circulating strains, including KP.2 and KP.3.
Subject to necessary permissions from the FDA and CDC, Novavax intends to market this updated COVID-19 vaccine in pre-filled syringes by mid-August. If authorized/approved, it will be the only protein-based non-mRNA COVID-19 vaccine in the United States.
Novavax's filing is based on guidance from the FDA issued last week, which advised COVID-19 vaccine manufacturers Moderna MRNA, Novavax and Pfizer PFE to update their respective COVID-19 vaccines to target the KP.2 strain if it is feasible. This guidance is an update on the FDA's prior advice issued at the beginning of this month that recommended updating the COVID-19 vaccines to target the JN.1 strain.
The FDA's prior advice was in line with the recommendation issued by a World Health Organization (WHO) advisory committee on Apr 26. However, the FDA pointed out that post the WHO recommendation, another subvariant named KP.2 has become the dominant strain in the country since the end of April. The latest CDC data (as of Jun 8) shows that the KP.2 and KP.3 strains were the most prevalent, accounting for nearly half of COVID-19 cases in the country.
The KP.2 strain is a JN.1-derived subvariant that contains two new additional mutations. Per the FDA, this subvariant provides an advantage to the virus ‘either in terms of fitness or escape from immunity.'
Management is also working with other regulatory authorities across the globe to secure authorization/approval for updating its protein-based COVID-19 vaccine.
Year to date, the stock has skyrocketed 200.0% against the industry's 6.3% fall.
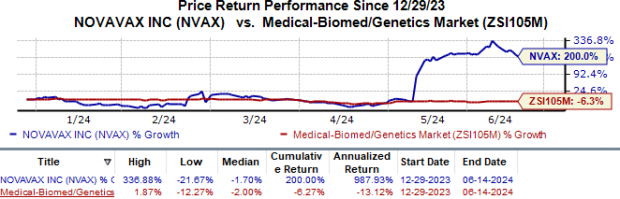
Image Source: Zacks Investment Research
Currently, Novavax's COVID-19 vaccine is only authorized by the FDA for use in individuals aged 12 years and older. The FDA is reviewing the company's regulatory filing seeking full approval for the COVID-19 vaccine. A final decision is expected before April 2025.
Earlier this month, Moderna submitted an application to the FDA seeking approval of its mRNA-based COVID-19 vaccine, for the 2024-2025 fall season. Pfizer is yet to provide an update on the FDA filing for its COVID-19 vaccine.
Compared with Pfizer and Moderna, Novavax was not able to reap the benefits of the pandemic due to a delayed launch of its COVID-19 vaccine. During last year's vaccination season, sales of Novavax suffered due to delayed approval of its vaccine formulation and product launch. Some investors and analysts believe that Novavax's early participation in the upcoming vaccination season could capitalize on the opportunity.
Starting next year, Sanofi SNY will gain rights to co-market Novavax's protein-based COVID-19 vaccine globally. The French drugmaker will also have the sole license to develop and market the Novavax vaccine in combination with its influenza vaccine. In return, Novavax will be eligible to receive up to $1.2 billion, including $500 million in upfront payment and the rest in milestone payments. NVAX will also be eligible to receive tiered double-digit percentage royalty payments on product sales under this deal. In addition, Sanofi also agreed to make an equity investment of nearly $70 million in Novavax in exchange for a 4.9% stake.
The deal with Sanofi breathes new life into Novavax, which had been facing uncertainties in its business for a long time. With the backing of a pharma giant, Novavax expects to increase the market share and presence of its COVID-19 vaccine to a larger audience. This deal even allowed management to do away with its previous concerns about its ability to continue as a ‘going concern.'
Using the funds from the Sanofi deal, Novavax also started making plans to expand its pipeline. Alongside its first-quarter earnings, management claimed to have been developing a new approach for vaccinating against H5N1 bird flu. It is also advancing core technology for different uses like mucosal vaccination and high-density nanoparticles.
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold).
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Comments
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
