Beam Therapeutics Inc. BEAM announced that it has dosed the first patient in the phase I/II study, evaluating its investigational in vivo base editing therapy, BEAM-302, for the treatment of alpha-1 antitrypsin deficiency (AATD).
The open-label, dose-escalation study will investigate the safety, pharmacodynamics, pharmacokinetics and efficacy of BEAM-302 for the given indication. The study is designed to identify the optimal dosage of BEAM-302.
AATD is an inherited genetic disorder that can cause early onset of emphysema and liver disease. Currently, there are no curative treatments approved for AATD.
BEAM received clearance for its clinical trial authorization application from the United Kingdom Medicines and Healthcare Products Regulatory Agency for BEAM-302 in March 2024.
Shares of Beam Therapeutics have declined 8.7% year to date compared with the industry's decrease of 4.6%.
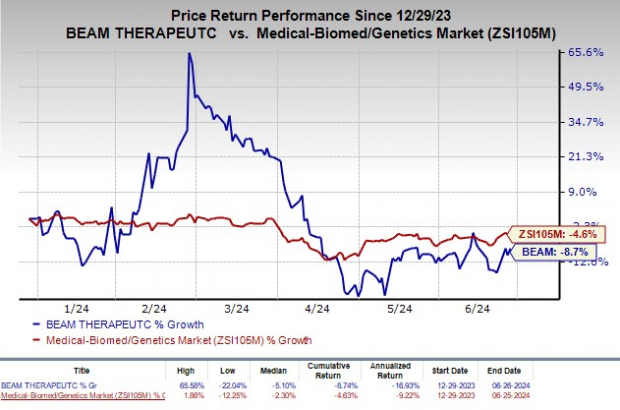
Image Source: Zacks Investment Research
BEAM is also developing another pipeline candidate in its genetic disease portfolio, BEAM-301. The company is looking to initiate a clinical study on BEAM-301 for the treatment of glycogen storage disease 1a in the United States. An investigational new drug application for BEAM-301 is expected to be filed shortly.
We remind investors that BEAM is developing its leading ex-vivo genome-editing candidate, BEAM-101, in the phase I/II BEACON study for the treatment of adult patients with sickle cell disease.
The company has completed dosing and engraftment for the three patients in the sentinel cohort of the BEACON study. Data from multiple patients in the study is expected in the second half of 2024.
Though still in the early stage, BEAM's pipeline holds great potential. If successfully developed and commercialized, BEAM-101 can boost the company's prospects in the days ahead.
Zacks Rank & Stocks to Consider
Beam Therapeutics currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Acrivon Therapeutics, Inc. ACRV, Aligos Therapeutics, Inc. ALGS and RAPT Therapeutics, Inc. RAPT, each carrying a Zacks Rank #2 (Buy) at present.
In the past 60 days, estimates for Acrivon Therapeutics' 2024 loss per share have narrowed from $3.16 to $2.47. Loss per share estimates for 2025 have narrowed from $3.07 to $2.55. Year to date, shares of ACRV have risen 17.9%.
ACRV's earnings beat estimates in three of the trailing four quarters and missed the same on the remaining one occasion, the average surprise being 3.56%.
In the past 60 days, estimates for Aligos Therapeutics' 2024 loss per share have narrowed from 84 cents to 73 cents, while loss per share estimates for 2025 have narrowed from 82 cents to 71 cents. Year to date, shares of ALGS have declined 40%.
ALGS's earnings beat estimates in three of the trailing four quarters and missed on the other occasion, the average surprise being 7.83%.
In the past 60 days, estimates for RAPT Therapeutics' 2024 loss per share have narrowed from $3.19 to $2.93. Loss per share estimates for 2025 have narrowed from $2.40 to $2.05. Year to date, shares of RAPT have plunged 87.7%.
RAPT's earnings beat estimates in two of the trailing four quarters while missing on the remaining two occasions, the average surprise being 3.19%.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.