Merck MRK announced that the U.S. Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices (ACIP) has unanimously voted to recommend MRK's newly approved 21-valent pneumococcal conjugate vaccine (PCV) Capvaxive. The FDA approved Capvaxive earlier this month for the prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults. Capvaxive became the first PCV specifically designed for adults to be approved by the FDA.
Capvaxive targets serotypes that account for approximately 84% of all invasive pneumococcal disease in older adults (50 years and older) in the United States, including eight serotypes not covered by currently licensed vaccines.
The ACIP recommends a single dose of Capvaxive for three distinct patient populations, the first being adults aged 65 years and older who have never received a pneumococcal conjugate vaccine or whose vaccination history is unknown. The second eligible patient population includes adults aged 19-64 years with certain underlying medical conditions or risk factors who have not previously received a pneumococcal conjugate vaccine or whose vaccination history is unknown.
The final patient population for whom ACIP recommends Merck's Capvaxive are adults aged 19 years and older who have begun their pneumococcal vaccine series with 13-valent PCV (PCV13) but have not received all the recommended pneumococcal 23-valent polysaccharide (PPSV23) vaccine doses.
Additionally, the ACIP advises that shared clinical decision-making should be applied for the use of a supplemental dose of Capvaxive for adults aged 65 years and older who have already completed their vaccine series with both PCV13 and PPSV23.
These new recommendations highlight Capvaxive's potential to enhance protection against pneumococcal disease in older adults and those with specific health conditions. However, these recommendations are provisional, which means they are not legally binding. These will only be official when the CDC reviews and finalizes the same.
Year to date, shares of MRK have jumped 19.1% compared with the industry's 21.9% growth.
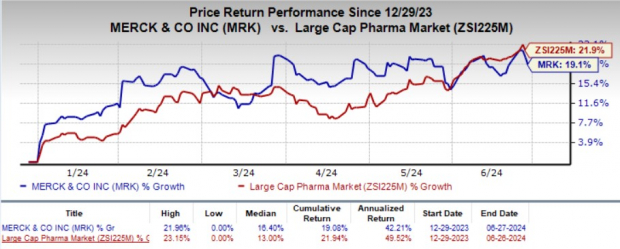
Image Source: Zacks Investment Research
Capvaxive's approval was based on data from four phase III studies conducted across a range of adult populations. In the studies, Capvaxive demonstrated robust immune responses in both vaccine-naïve and vaccine-experienced adult populations.
Merck also has a 15-valent PCV called Vaxneuvance (V114) in its vaccine portfolio that was approved in 2021. Vaxneuvance generated sales of $665 million in 2023, down 2% year over year.
Zacks Rank and Stocks to Consider
Merck currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ALX Oncology Holdings, Annovis Bio ANVS and Compugen CGEN, each carrying a Zacks Rank #2 (Buy) at present.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology's 2024 loss per share has remained constant at $2.89. During the same period, the consensus estimate for 2025 loss per share has remained constant at $2.73. Year to date, shares of ALXO have plunged 60.8%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
In the past 30 days, the Zacks Consensus Estimate for Annovis' 2024 loss per share has remained constant at $2.46. During the same period, the consensus estimate for 2025 loss per share has remained constant at $1.95. Year to date, shares of ANVS have plunged 67.9%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
In the past 30 days, the Zacks Consensus Estimate for Compugen's 2024 earnings per share has remained constant at 5 cents. The consensus estimate for 2025 loss per share is currently pegged at 11 cents. Year to date, shares of CGEN have lost 11.1%.
CGEN's earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 5.79%.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
