uniQure N.V. QURE surged 76.5% on Jul 9 after it announced updated interim data, including up to 24 months of follow-up data, from 29 treated patients enrolled in the ongoing early to mid-stage studies of AMT-130 for Huntington's disease in the United States and EU. Results demonstrated a potential long-term, durable clinical benefit and reduction of a key marker of neurodegeneration upon treatment with the candidate.
AMT-130, a one-time administered investigational gene therapy, is being developed to treat Huntington's disease. Huntington's disease is a genetic disorder that causes the progressive breakdown of nerve cells in the brain, which leads to a decline in cognitive and physical abilities, often resulting in movement, thinking and psychiatric problems.
uniQure is simultaneously conducting two phase I/II studies of AMT-130, with 26 participants in the United States and 13 in the EU/UK to treat Huntington's disease. Among these, a total of 29 patients received either a low dose (12 patients) or a high dose (17 patients) of AMT-130, while 10 control patients underwent imitation surgery. As of Mar 31, 2024, follow-up data at 24 months were available for 21 patients, including 12 from the low-dose group and nine from the high-dose group.
Please note that uniQure, for the first time, performed a statistical analysis of clinical outcomes at 24 months for the 21 treated patients. This analysis, compared with an expanded, propensity-weighted external control group of 154 patients, developed in collaboration with the Cure Huntington's Disease Initiative. The control data was derived from the TRACK-HD, TRACK-ON and PREDICT-HD natural history studies.
Year to date, shares of QURE have lost 1.5% compared with the industry's 6.3% decline.
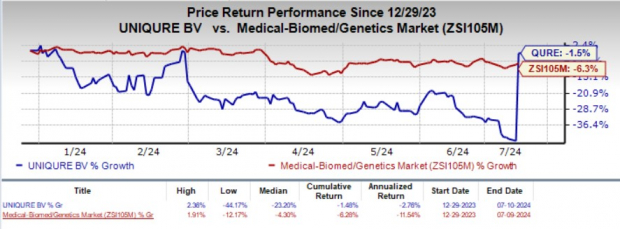
Image Source: Zacks Investment Research
Per the data readout from the phase I/II Huntington's disease studies, patients treated with the high dose of AMT-130 experienced a statistically significant, dose-dependent slowing in disease progression of 80%, as measured by cUHDRS after 24 months. For the low-dose group, a 30% slowing of disease progression, as measured by cUHDRS, was observed compared with the control group at month 24.
Please note that the cUHDRS has been demonstrated to be the most sensitive measurement of clinical progression in Huntington's disease patients.
Patients receiving the high dose of AMT-130 also showed near-baseline stability in motor and cognitive function throughout the 24 months of follow-up. Additionally, a statistically significant reduction of Neurofilament light chain (NfL) protein in cerebrospinal fluid (CSF) was observed in patients treated with AMT-130.
These patients had a mean reduction in CSF NfL of 11% compared with baseline at 24 months. Treatment with both dose strengths of AMT-130 resulted in mean CSF NfL levels below baseline at 24 months.
CSF NfL is an important biomarker of neurodegeneration and strongly associated with the clinical severity of Huntington's disease. An independent natural history study showed a 26% increase in CSF NfL at 24 months in patients with early manifest Huntington's disease.
Per the updated data, AMT-130 continues to be generally well-tolerated with a safety profile that is manageable at both doses. No new drug-related serious adverse events were reported.
We remind the investors that uniQure enjoys the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation for AMT-130 to treat Huntington's disease.
The company is currently gearing up to hold an RMAT meeting with the FDA in the second half of 2024 to discuss these updated positive data seeking potential expedited clinical development pathways and accelerated approval for AMT-130.
In the second half of 2024, the company expects to complete enrolling patients in the third cohort of the U.S. phase I/II study exploring AMT-130 in combination with immunosuppression. Safety data from this cohort is anticipated in the first half of 2025.
Additionally, uniQure also shared its plan to present another interim analysis from the ongoing phase I/II studies of AMT-130 to treat Huntington's disease in mid-2025. The data from this interim analysis will include a 36-month comparison of treated patients to the propensity score-weighted external control.
Apart from AMT-130, uniQure's wholly-owned clinical pipeline comprises several other candidates that are currently undergoing early-stage development for the treatment of patients with refractory temporal lobe epilepsy, amyotrophic lateral sclerosis and Fabry disease.
Please note that the company also markets an internally developed gene therapy for the treatment of hemophilia B in the United States and EU under the brand name Hemgenix. The approvals in the U.S. and EU markets in 2022 and 2023, respectively, marked a significant milestone in the field of genomic medicine as it brought a new treatment approach for patients living with hemophilia.
Zacks Rank and Other Stocks to Consider
uniQure currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks from the drug/biotech industryare ALX Oncology Holdings ALXO, Annovis Bio ANVS and Compugen CGEN, each carrying a Zacks Rank #2 at present.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology's 2024 loss per share has remained constant at $2.89. During the same period, the consensus estimate for 2025 loss per share has remained constant at $2.73. Year to date, shares of ALXO have plunged 57.3%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
In the past 30 days, the Zacks Consensus Estimate for Annovis' 2024 loss per share has remained constant at $2.46. During the same period, the consensus estimate for 2025 loss per share has remained constant at $1.95. Year to date, shares of ANVS have lost 22.1%.
ANVS beat estimates in three of the trailing four quarters and missed once, delivering an average negative surprise of 1.39%.
In the past 30 days, the Zacks Consensus Estimate for Compugen's 2024 earnings per share has remained constant at 5 cents. The consensus estimate for 2025 loss per share is currently pegged at 11 cents. Year to date, shares of CGEN have lost 11.1%.
CGEN's earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 5.79%.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.