Vanda Pharmaceuticals Inc. VNDA announced that the FDA issued a complete response letter to its new drug application (NDA) seeking approval for its pipeline candidate, tradipitant for the treatment of symptoms in gastroparesis.
Shares of the company were down 6.1% on Sept. 19 following the announcement of the news.
Marked by delayed gastric emptying, gastroparesis is a serious condition that slows down the stomach's ability to empty its contents. The FDA has not approved any effective medicine for treating gastroparesis in more than 40 years.
Year to date, shares of Vanda have increased 10.2% compared with the industry's rise of 0.4%.
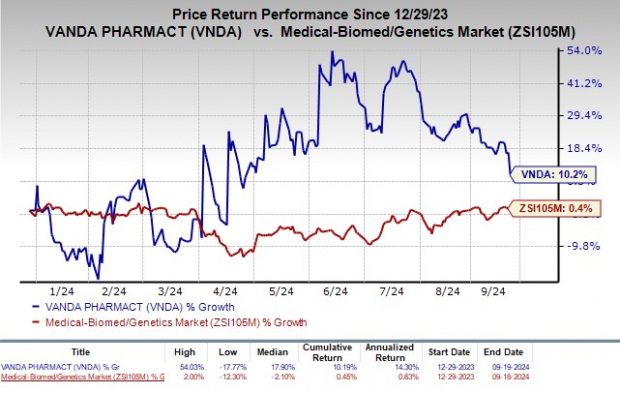
Image Source: Zacks Investment Research
More on FDA's CRL for VNDA's Tradipitant
Per the CRL, the FDA called for Vanda to conduct additional studies on tradipitant with a design that was inconsistent with the advice of key experts in the field and not appropriate based on the scientific understanding of the disease.
Also, per management, the FDA delayed its decision by more than 185 days and failed to meet the requirements specified by the Food Drug and Cosmetic Act (FDCA).
Notably, the FDCA requires that the FDA review an NDA and provide either an approval or a possible hearing within 180 days of the filing. The FDA failed to do either.
Per the company, it had time and again asked the FDA to hold an advisory committee meeting to discuss the NDA for tradipitant while the regulatory body declined.
Several patients treated with tradipitant have now filed a Citizen Petition pleading with the regulatory body to approve tradipitant for the treatment of gastroparesis.
VNDA's Development Plans for Tradipitant
Despite the FDA declining to approve tradipitant for treating symptoms in gastroparesis, the company will continue to pursue the marketing approval for tradipitant in the given indication.
Apart from gastroparesis, VNDA is also developing tradipitant for preventing vomiting induced by motion sickness.
In May 2024, the company announced positive data from a second phase III study that investigated tradipitant for preventing vomiting induced by motion sickness.
Vanda plans to submit an NDA for tradipitant to the FDA for the treatment of motion sickness later in 2024.
Zacks Rank & Stocks to Consider
Vanda currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the biotech sector are Illumina, Inc. ILMN, Krystal Biotech, Inc. KRYS and Fulcrum Therapeutics, Inc. FULC, each sporting a Zacks Rank #1 (Strong Buy) at present.
In the past 60 days, estimates for Illumina's 2024 earnings per share have moved up from $1.84 to $3.63. Earnings per share estimates for 2025 have improved from $3.22 to $4.43. Year to date, shares of ILMN have lost 3.5%.
ILMN's earnings beat estimates in each of the trailing four quarters, with the average surprise being 463.46%.
In the past 60 days, estimates for Krystal Biotech's 2024 earnings per share have increased from $2.09 to $2.38. Earnings per share estimates for 2025 have improved from $4.33 to $7.31. Year to date, shares of KRYS have risen 48.7%.
KRYS' earnings beat estimates in three of the trailing four quarters while missing on the remaining occasion, with the average surprise being 45.95%.
In the past 60 days, estimates for Fulcrum Therapeutics' 2024 loss per share have narrowed from $1.33 to 28 cents. Loss per share estimates for 2025 have narrowed from $1.71 to $1.14. Year to date, shares of FULC have plunged 48.9%.
FULC's earnings beat estimates in each of the trailing four quarters, with the average surprise being 393.18%.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.