Sage Therapeutics, Inc. SAGE announced that partner Biogen BIIB has terminated its rights related to the investigational neurology candidate, SAGE-324. A phase II study on SAGE-324 for treating essential tremor failed to meet the primary endpoint in July.
The termination will be effective from Feb. 17, 2025.
Following the termination of the deal by BIIB, Sage Therapeutics will obtain full ownership and rights of SAGE-324. The company plans to study SAGE-324 for other indications, if any.
Year to date, shares of Sage Therapeutics have plunged 66.9% compared with the industry's decrease of 1.9%.
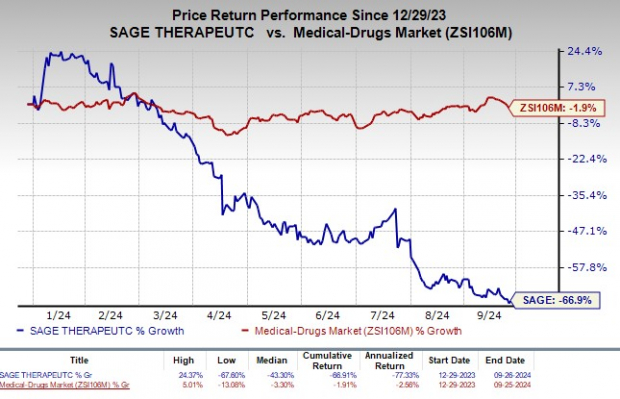
Image Source: Zacks Investment Research
SAGE's Licensing Agreement With BIIB
In July, Sage Therapeutics and Biogen announced top-line data from the phase II KINETIC 2 study, which evaluated SAGE-324 as a potential treatment for ET. The study failed to demonstrate a statistically significant dose-response relationship on the primary endpoint in participants with ET.
Based on the disappointing data, SAGE and BIIB decided to stop further clinical development of SAGE-324 in ET.
SAGE currently markets the depression drug Zurzuvae (zuranolone) in partnership with Biogen.
Zurzuvae, the first and only oral treatment indicated for adults with postpartum depression (PPD), was approved by the FDA in August 2023 and commercially launched in December.
Sage Therapeutics and Biogen, equally share profits and losses for the commercialization of Zurzuvae in the United States. In ex-U.S. markets, Biogen records product sales (excluding Japan, Taiwan and South Korea) and pays royalties to Sage Therapeutics.
Sage Therapeutics and BIIB are focused on establishing Zurzuvae as a first-line therapy and the standard of care for women with PPD.
The FDA issued a complete response letter for the new drug application (NDA) for zuranolone for treating adults with major depressive disorder in August 2023. Per the FDA, the data supporting the NDA filing did not provide substantial evidence of effectiveness to support a potential approval. It recommended conducting additional clinical studies.
The company is evaluating the necessary steps to address this CRL.
SAGE's Zacks Rank & Stocks to Consider
Sage Therapeutics currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Krystal Biotech, Inc. KRYS and Fulcrum Therapeutics, Inc. FULC, each sporting a Zacks Rank #1 (Strong Buy) at present.
In the past 60 days, estimates for Krystal Biotech's 2024 earnings per share have increased from $2.09 to $2.38. Earnings per share estimates for 2025 have improved from $4.33 to $7.31. Year to date, shares of KRYS have risen 43.8%.
KRYS' earnings beat estimates in three of the trailing four quarters while missing on the remaining occasion, with the average surprise being 45.95%.
In the past 60 days, estimates for Fulcrum Therapeutics' 2024 loss per share have narrowed from $1.33 to 28 cents. Loss per share estimates for 2025 have narrowed from $1.71 to $1.14. Year to date, shares of FULC have plunged 40.8%.
FULC's earnings beat estimates in each of the trailing four quarters, with the average surprise being 393.18%.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.