Alnylam Pharmaceuticals ALNY announced that it has submitted a Type II Variation to the European Medicines Agency for vutrisiran, an investigational RNAi therapeutic being developed to treat ATTR amyloidosis with cardiomyopathy (ATTR-CM). Shares of the company gained 4.6% in response to the encouraging news.
Please note that Alnylam already markets vutrisiran in the EU under the brand name Amvuttra for treating hereditary transthyretin-mediated (hATTR) amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy.
The application seeking approval for vutrisiran to treat ATTR-CM is supported by positive results from ALNY's pivotal phase III HELIOS-B study, which met all primary and secondary endpoints across both the overall and monotherapy populations, each with statistical significance.
Year to date, shares of Alnylam have gained 57% against the industry's 1% decline.
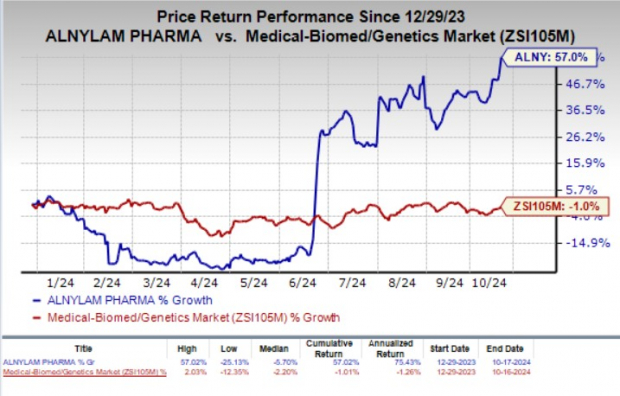
Image Source: Zacks Investment Research
Per the data readout, vutrisiran reduced mortality and cardiovascular events while improving functional capacity (measured by the six-minute walk test), quality of life (using the Kansas City Cardiomyopathy Questionnaire), and heart failure symptoms and severity (via New York Heart Association class) in ATTR-CM patients.
Additionally, the safety profile of vutrisiran in the HELIOS-B study was consistent with its known profile for treating hATTR amyloidosis with polyneuropathy in adults. In the late-stage study, the rates of adverse events, including those leading to discontinuation, were similar between the vutrisiran and placebo groups.
Amvuttra – The Key Growth Driver for Alnylam
Please note that Alnylam also markets Amvuttra, the company's lead drug, in the United States for the treatment of adult patients with polyneuropathy of hATTR amyloidosis. The drug's sales contribute significantly to ALNY's top line. Amvuttra generated sales worth $425.4 million in the first half of 2024, up 82% year over year on a reported basis. The encouraging uptake of the drug is driven by new patients starting treatment as well as patients switching from Onpattro. Onpattro is approved in the United States and EU to treat hATTR amyloidosis in adults.
We remind the investors that a supplemental new drug application for vutrisiran, seeking its label expansion to treat ATTR-CM is also currently under review in the United States. If the regulatory applications in the EU and the United States are approved, Alnylam believes that vutrisiran has the potential to become the new standard of care for the treatment of ATTR-CM. This will expand the eligible patient population for the drug driving substantial growth for the company in the future.
Other marketed products include Givlaari for acute hepatic porphyria and Oxlumo injection for subcutaneous use for the treatment of primary hyperoxaluria type 1 to lower urinary and plasma oxalate levels in pediatric and adult patients.
Alnylam also markets a fifth drug, Leqvio (inclisiran), in collaboration with Novartis NVS to treat hypercholesterolemia in the EU. In the United States, it is approved to reduce low-density lipoprotein cholesterol (LDL-C) with two doses per year. Per the agreement, Alnylam has granted Novartis exclusive and worldwide rights to manufacture and commercialize RNAi therapeutics targeting PCSK9 for treating hypercholesterolemia and other human diseases, including Leqvio. Novartis has also received FDA approval to expand Leqvio's label to include earlier use in patients with elevated LDL-C who have an increased risk of heart disease, as an adjunct to diet and statin therapy.
ALNY's Zacks Rank and Other Stocks to Consider
Alnylam currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the biotech sector are ANI Pharmaceuticals ANIP and Amicus Therapeutics FOLD. While ANIP sports a Zacks Rank #1 (Strong Buy), FOLD carries a Zacks Rank #2 at present.
In the past 60 days, estimates for ANI Pharmaceuticals' 2024 earnings per share have moved up from $4.69 to $4.81. EPS estimates for 2025 have improved from $5.37 to $5.86, during the same period. Year to date, shares of ANIP have gained 8.2%.
ANI Pharmaceuticals' earnings beat estimates in each of the trailing four quarters, with the average surprise being 31.32%.
In the past 60 days, estimates for Amicus' 2024 EPS have remained constant at 20 cents. EPS estimates for 2025 have increased from 48 cents to 50 cents, during the same period. Year to date, shares of FOLD have lost 26%.
Amicus' earnings beat estimates in three of the four trailing four quarters, missing the mark once, with the average surprise being 23.96%.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
