This article was originally published on Psychedelic Spotlight and appears here with permission.
If you fail to meet even one of the inclusion criteria, or you meet even one of the exclusion criteria, then you are not eligible and you will not be selected for participation.
It seems like every week there is a new psychedelic clinical trial showing the potential for psychedelic-assisted therapy, using compounds such as psilocybin, LSD or MDMA, to heal mental health conditions like depression, anxiety or PTSD.
Given this exciting new reality, it is only natural that many people are asking: “How can I receive these psychedelic-assisted therapies to help with my mental health.”
Unfortunately, however, the answer is that access is still quite limited. Ketamine-therapy is currently allowed off-label, but for any other psychedelic such as MDMA or psilocybin, basically, if you live in the USA, Canada or Europe, the only option is to enroll in a clinical trial. By the end of next year, Oregon is set to legalize psilocybin-assisted therapy for everyone, and in the United States, MDMA-therapy for PTSD will likely be legalized, but for now, enrolling in a clinical trial is basically the only way to legally receive a psychedelic-therapy.
So, given that, the best question to ask is, “How do I enroll in a psychedelic clinical trial?”
In this article I will endeavor to answer that question in 5 easy steps.
Step 1: Be Diagnosed
In order to join a clinical trial, you MUST have whichever condition the trial is attempting to treat.
For example there are many clinical trials attempting to treat Major Depressive Disorder with psychedelic-assisted therapy, using psilocybin, ketamine, MDMA, LSD, and more. And obviously, in order for the treatment data to be accurate, all participants must have been diagnosed by a doctor. So, if you want to join a trial treating Major Depressive Disorder, you actually have to be diagnosed with Major Depressive Disorder, you can’t just feel depressed. The same goes for trials testing treating Generalized Anxiety Disorder, PTSD, ADHD, etc.
Now, if you are not diagnosed with a mental health condition, that does not mean that there is zero chance of becoming a participant in a clinical trial. Phase 1 trials test the safety of drugs in healthy humans, before moving on to test their efficacy in Phase 2 and 3 trials. So if you have no mental health conditions and are physically healthy—especially when it comes to cardio-vascular health— chances are you can apply to be a participant in a Phase 1 clinical trial.
Step 2: Find a Clinical Trial Near You
Despite there being more psychedelic clinical trials than ever, with dozens currently recruiting, on the scale of billions of people, there are still relatively few. Therefore, if you want to be enrolled in a clinical trial, you have to be a little lucky. If you live in a major city center, such as New York City, Montreal or London, it is much more likely that there is a clinical trial near you.
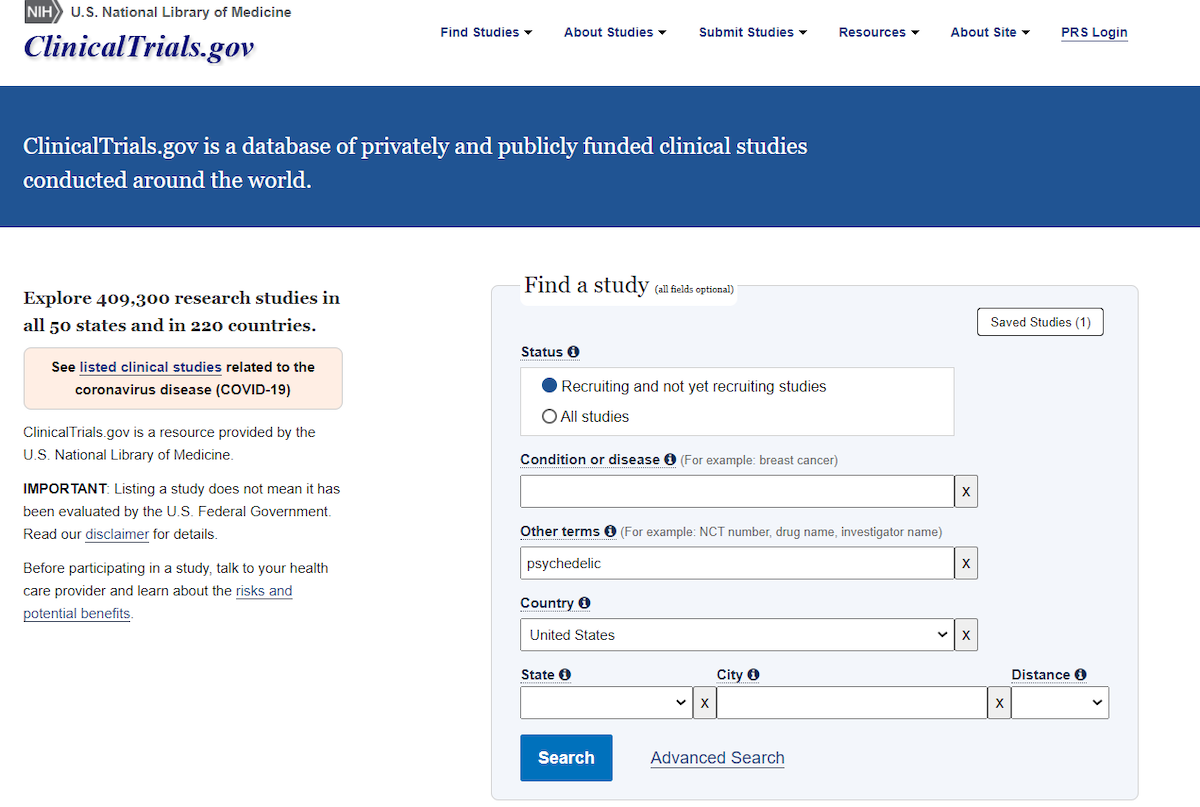
Here is a list of clinical trials currently recruiting. It is updated regularly, so check back every so often.
You can also use the website ClinicalTrial.gov. Just select “recruiting and not yet recruiting studies,” enter a condition if you are looking to treat a specific issue—for example “Major Depressive Disorder”— an “other term” such as psilocybin or psychedelic, and a geographic location. Note, you do not need to enter all fields. For example, selecting “recruiting and not yet recruiting studies,” using the term “psychedelic” and the geographic location of the United States, I found 66 trials.
Step 3: Ensure You Meet ALL the Requirements
Once you select a clinical trial in your area which is attempting to treat the condition you have been diagnosed for—or if its a Phase 1 and you are healthy—you must ensure that you meet ALL of the inclusion criteria, and NONE of the exclusion criteria.
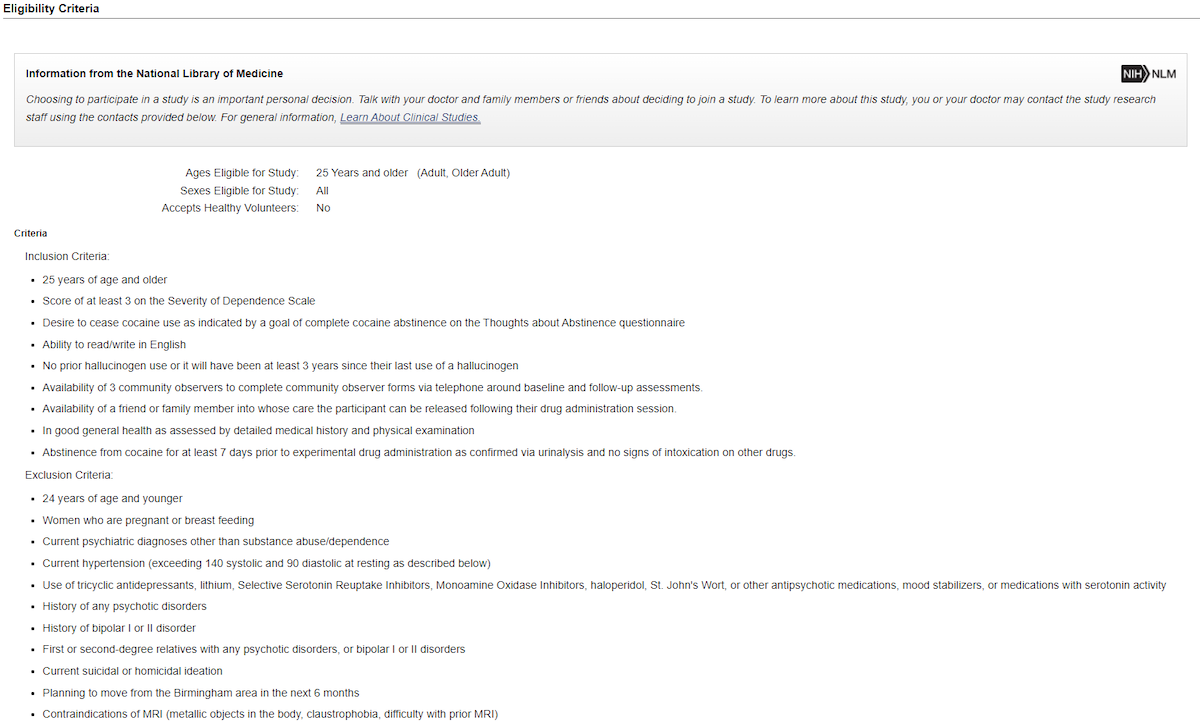
For example, using ClinicalTrials.gov, I selected a Phase 2 trial called Psilocybin-facilitated Treatment for Cocaine Use. Scrolling down past the study description, design, arms and interventions, and outcome measures, you will find the Eligibility Criteria. In this case, there are 9 inclusion criteria, and 11 exclusion criteria.
Some of the inclusion criteria are: you must be 25 years of age and older; have a score of at least 3 on the Severity of Dependence Scale; have a desire to cease cocaine use as indicated by a goal of complete cocaine abstinence on the Thoughts about Abstinence questionnaire; and have the ability to read/write in English. Note, you do not necessarily need to have completed these questionnaires in the past. You may have the chance to complete them in the screening process.
Some of the exclusion criteria are: current psychiatric diagnoses other than substance abuse/dependence; a history of any psychotic disorders; a history of bipolar I or II disorder; and current suicidal or homicidal ideation.
If you fail to meet even one of the inclusion criteria, or you meet even one of the exclusion criteria, then you are not eligible and you will not be selected for participation. So make sure you read the inclusion and exclusion criteria carefully to make sure you don’t waste your time, or the time of the researchers.
Step 4: Apply
Once you have ensured that you are eligible for a trial near you, it is time to apply. Though the exact process can differ, generally the first step is to reach out to those in charge of recruiting.
Using the example of the trial treating cocaine addiction with psilocybin above, if you scroll down the page to the “Contacts and Locations” section, you will see the contact details of the person in charge of recruiting. After you make initial contact, a dialogue will commence between you and the organizers.
Step 5: Complete the Screening Process
After initial contact, the researchers will make sure that you meet the eligibility criteria and that you are a good fit for their trial. An appointment will likely be set up for you to meet them, where you will undergo physical and mental exams, and other eligibility tests.
Assuming that you do meet all of the requirements, and the researchers believe you to be a good fit for the trial, you will have to give informed consent. The team will make sure you understand the protocol, your responsibilities, the potential risks involved, what will happen with the data collected from the study and any other pertinent information. If you consent to all of the terms, you will have to sign a consent form to show that you understand what you are volunteering for.
Even if you originally consent, it is important to know that at any time you can still choose to leave the trial, even if you had previously given consent.
If you have made it through all of these steps, then it is time to start the trial.
It is important to note that most trials have a “placebo” arm, meaning that a portion of the trial volunteers will not receive the experimental medicine, but rather a sugar pill or some other form of inactive compound, such as saline. In some trials this will be half of the participants, and in others it will be 20% or some other proportion. Sometimes those who receive the placebo originally will be offered the chance to later receive the medicine, but not always. So understand this before applying.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
Cannabis is evolving – don’t get left behind!
Curious about what’s next for the industry and how to leverage California’s unique market?
Join top executives, policymakers, and investors at the Benzinga Cannabis Market Spotlight in Anaheim, CA, at the House of Blues on November 12. Dive deep into the latest strategies, investment trends, and brand insights that are shaping the future of cannabis!
Get your tickets now to secure your spot and avoid last-minute price hikes.

