Zinger Key Points
- Dept. of Justice (DOJ) proposes rescheduling marijuana to Schedule III under the Controlled Substances Act.
- Dept of Health and Human Services cites lower abuse potential, accepted medical use for change.
- DOJ opens 60-day public comment period; significant policy shift expected.
- Markets are swinging wildly, but for Matt Maley, it's just another opportunity to trade. His clear, simple trade alerts have helped members lock in gains as high as 100% and 450%. Now, you can get his next trade signal—completely free.
The Department of Justice (DOJ) and the Drug Enforcement Administration (DEA) have proposed a groundbreaking change to the federal scheduling of marijuana, aiming to reclassify it from Schedule I to Schedule III of the Controlled Substances Act (CSA). This move, detailed in a formal notice of proposed rulemaking, reflects the evolving scientific understanding and legal landscape surrounding marijuana, acknowledging its medical use and comparatively lower potential for abuse.
Historic Shift In Marijuana Policy
Initially classified as a Schedule I substance in 1970, marijuana has been a focal point of debate and petition for rescheduling. The last major review in 2016 upheld its Schedule I status, citing high potential for abuse and no accepted medical uses. However, the legal landscape has significantly changed, with 38 U.S. states and several territories legalizing medical marijuana, prompting a reexamination by federal agencies.
Timeline Of Marijuana's Federal Scheduling History
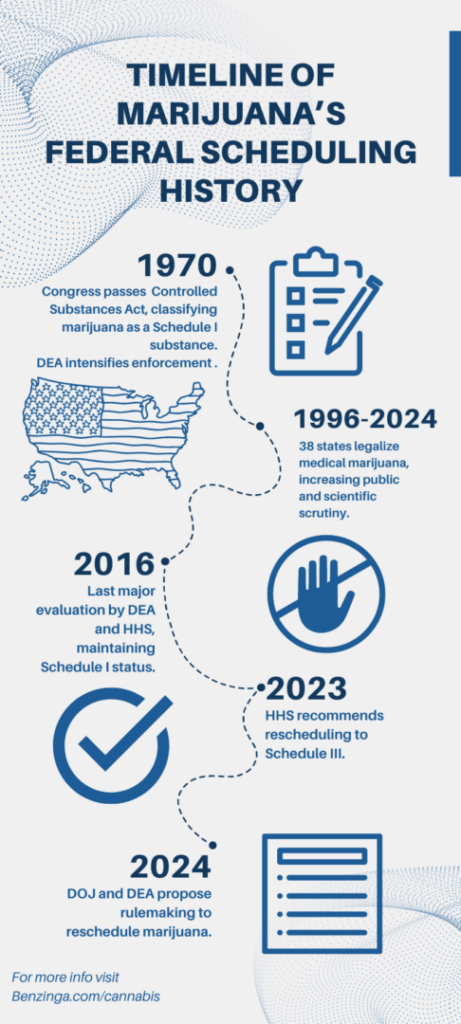
To use this infographic, please link to Benzinga.com/cannabis
Key Points From The Proposal
The Department of Health and Human Services (HHS) conducted a comprehensive evaluation, leading to the recommendation to reschedule marijuana based on the following eight factors:
- Potential for Abuse:
- Lower potential for abuse compared to substances in Schedule I and II.
- Epidemiological data shows lower rates of adverse outcomes compared to heroin and cocaine.
- Pharmacological Effects:
- Δ9-THC acts on CB1 and CB2 receptors in the brain and peripheral tissues.
- Produces both rewarding (euphoria) and adverse effects (sedation, anxiety).
- Current Scientific Knowledge:
- Significant variation in chemical profiles across different marijuana strains.
- Increased potency over the past few decades.
- History and Pattern of Abuse:
- Widespread use for medical and recreational purposes.
- Lower incidence of severe medical outcomes compared to other controlled substances.
- Scope and Significance of Abuse:
- Abuse produces clear harmful consequences but less severe than other substances.
- Risk to Public Health:
- Public health risks are lower compared to drugs like heroin and cocaine.
- Dependence Liability:
- Moderate or low physical dependence and high psychological dependence.
- Immediate Precursor:
- Marijuana is not an immediate precursor of a controlled substance.
Proposal Details
- Regulatory Controls: Rescheduling to Schedule III would impose regulatory controls and restrictions applicable to Schedule III substances.
- Public Participation: Comments and requests for hearings must be submitted electronically or postmarked within 60 days of the publication in the Federal Register.
Implications Of Rescheduling
Medical Research and Use:
- Enhanced ability for researchers to study marijuana's therapeutic potential.
- More robust data on efficacy and safety could lead to new medical applications.
Legal and Regulatory Landscape:
- State-legal marijuana programs would remain, but with new federal regulations.
- Potential for changes in how marijuana-related offenses are prosecuted.
Economic Impact:
- Shift in market dynamics for marijuana-related businesses.
- Potential increase in investment and development within the cannabis industry.
Public Participation And Next Steps
The DOJ and DEA are actively seeking public comments and requests for hearings to ensure a comprehensive evaluation. The proposal outlines a detailed process for submitting feedback, emphasizing the importance of public involvement.
Submission Guidelines:
- Electronic Comments: Submit through the Federal eRulemaking Portal at regulations.gov.
- Paper Comments: Mail to DEA Federal Register Representative at 8701 Morrissette Drive, Springfield, Virginia.
- Hearing Requests: Send to the DEA Administrator at the same address, with a courtesy copy to the Hearing Clerk/OALJ.
Key Differences Between Schedule I And Schedule III
| Criteria | Schedule I | Schedule III |
|---|---|---|
| Potential for Abuse | High | Lower than Schedule I and II |
| Accepted Medical Use | No | Yes |
| Dependence Risk | High | Moderate to low physical, high psychological |
| Examples | Heroin, LSD, Ecstasy | Anabolic steroids, ketamine, codeine |
Eight-Factor Analysis Summary
| Factor | Details |
|---|---|
| Potential for Abuse | Lower compared to Schedule I and II substances |
| Pharmacological Effects | Δ9-THC acts on CB1 and CB2 receptors, produces euphoria and anxiety |
| Current Scientific Knowledge | Variable chemical profiles, increased potency over time |
| History and Pattern of Abuse | Widespread use, lower incidence of severe outcomes |
| Scope and Significance of Abuse | Harmful consequences less severe than other controlled substances |
| Risk to Public Health | Lower compared to drugs like heroin and cocaine |
| Dependence Liability | Moderate to low physical, high psychological |
| Immediate Precursor | Not an immediate precursor |
The proposed rescheduling of marijuana to Schedule III represents a pivotal moment in federal drug policy, potentially transforming the landscape for medical research, legal regulations, and the cannabis industry. Public participation is crucial in shaping the final decision, marking a significant step towards aligning federal regulations with evolving scientific and societal views on marijuana.
This content was partially produced with the help of AI tools and was reviewed and published by Benzinga editors. Lead image generated by AI. Infographic by Javier Hasse.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
Cannabis is evolving—don’t get left behind!
Curious about what’s next for the industry and how to stay ahead in today’s competitive market?
Join top executives, investors, and industry leaders at the Benzinga Cannabis Capital Conference in Chicago on June 9-10. Dive deep into market-shaping strategies, investment trends, and brand-building insights that will define the future of cannabis.
Secure your spot now before prices go up—this is where the biggest deals and connections happen!