Pfizer PFE announced that a phase III study on its mini-dystrophin gene therapy fordadistrogene movaparvovec for treating Duchenne muscular dystrophy ("DMD") failed to meet the primary endpoint as well as key secondary endpoints.
The global phase III study, CIFFREO is investigating fordadistrogene movaparvovec in patients 4-8 years of age with DMD. A progressive and degenerative disorder, DMD leads to weakness and wasting away of the body's muscles due to disruption of dystrophin production. The disease usually affects boys and is mostly diagnosed in early childhood.
The primary endpoint of the study was an improvement in motor function among the DMD boys treated with fordadistrogene movaparvovec compared to placebo. The primary endpoint was assessed by change in the North Star Ambulatory Assessment (NSAA) one year after treatment. NSAA is a rating scale used to measure motor abilities. The study also failed to show a significant difference between participants treated with fordadistrogene movaparvovec and placebo for the key secondary endpoints. The study's key secondary endpoints included 10-meter run/walk velocity and time to rise from floor velocity.
As regards the safety profile of the gene therapy, the adverse events were mostly mild to moderate in nature while the treatment-related serious adverse events responded to clinical management. Pfizer plans to share more detailed results from the study at an upcoming medical meeting.
Year to date, Pfizer's stock has declined 30.7% against an increase of 28.0% for the industry.
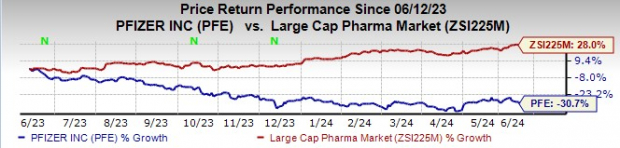
Image Source: Zacks Investment Research
Pfizer has, in the past, faced a couple of setbacks related to its clinical program on fordadistrogene movaparvovec.
In 2021, Pfizer paused the CIFFREO study after the FDA placed a clinical hold on the company's investigational new drug application for the program after a non-ambulatory participant died in the phase I study on the candidate. The FDA lifted the clinical hold in 2022, and the study resumed.
In May 2024, Pfizer reported the death of a young boy who participated in another phase II study called DAYLIGHT evaluating fordadistrogene movaparvovec for treating DMD in boys aged 2-3 years. The boy received the gene therapy a year back in early 2023. Pfizer said it did not have complete information about whether the death was caused due to the drug and said back then that it was working on learning more details. Following the death in the DAYLIGHT study. Pfizer paused dosing in the crossover arm of the CIFFREO study.
At present, Sarepta Therapeutics SRPT is the market leader in the treatment of DMD. Sarepta's commercial portfolio comprises four drugs for DMD, Exondys 51, Vyondys 53 (golodirsen), Amondys 45 (casimersen) and Elevidys (SRP-9001). Sarepta's Elevidys is the first and only one-shot gene therapy approved on an accelerated basis by the FDA in June 2023 for treating DMD in the United States. Elevidys achieved nearly $134.0 million in net product revenues in the first quarter of 2024. Sarepta has filed an efficacy supplement to the biologics license application (BLA) to expand the labeled indication of Elevidys and also to convert the accelerated approval to a full one. The FDA decision is expected on Jun 21, 2024.
PTC Therapeutics PTCT also markets two drugs for treating DMD — Translarna (approved in the European Union) and Emflaza (approved in the United States). PTC Therapeutics' DMD sales were $161 million in the first quarter of 2024
Earlier this week, Capricor Therapeutics CAPR announced the successful completion of a Type-B meeting with the FDA related to the BLA filing for its lead pipeline candidate, Deramiocel/CAP-1002 for DMD. The FDA agreed to a pre-BLA meeting based on their review of data from the HOPE -3 pivotal study. Capricor Therapeutics will now begin a rolling BLA filing for Deramiocel in the third quarter of 2024. Final data from the HOPE-3 study is expected in the fourth quarter of 2024
Several other companies like Dyne Therapeutics, Solid Biosciences and Wave Life Sciences, among others, are developing therapies for DMD.
Zacks Rank
Pfizer has a Zacks Rank #3 (Hold) currently.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
date | ticker | name | Price Target | Upside/Downside | Recommendation | Firm |
|---|
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
