Zinger Key Points
- Novavax has filed for authorization with Health Canada for its updated 2024-2025 protein-based COVID-19 vaccine.
- Positive news and regulatory progress have led to a significant increase in trading volume and investor optimism.
- Don't face extreme market conditions unprepared. Get the professional edge with Benzinga Pro's exclusive alerts, news advantage, and volatility tools at 60% off today.
Novavax, Inc. NVAX shares are moving higher on Monday. Here’s what you need to know.
What To Know: The company announced on July 2 that it has filed for authorization with Health Canada for its updated 2024-2025 protein-based COVID-19 vaccine (NVX-CoV2705) for individuals aged 12 and older. If approved, this would be the only protein-based COVID-19 vaccine option available in Canada. Novavax's updated vaccine targets the JN.1 variant and has shown broad neutralization responses against multiple variant strains, which could make it a significant option for public health programs.
What Else: Novavax has also submitted filings with the U.S. FDA and EMA and is working with other regulatory authorities globally to gain authorization for its updated vaccine. These efforts are aligned with the recommendations from the National Advisory Committee on Immunization (NACI), U.S. FDA, EMA and WHO regarding the vaccine composition. The company aims to have the vaccine available in Canada for immediate release post-authorization.
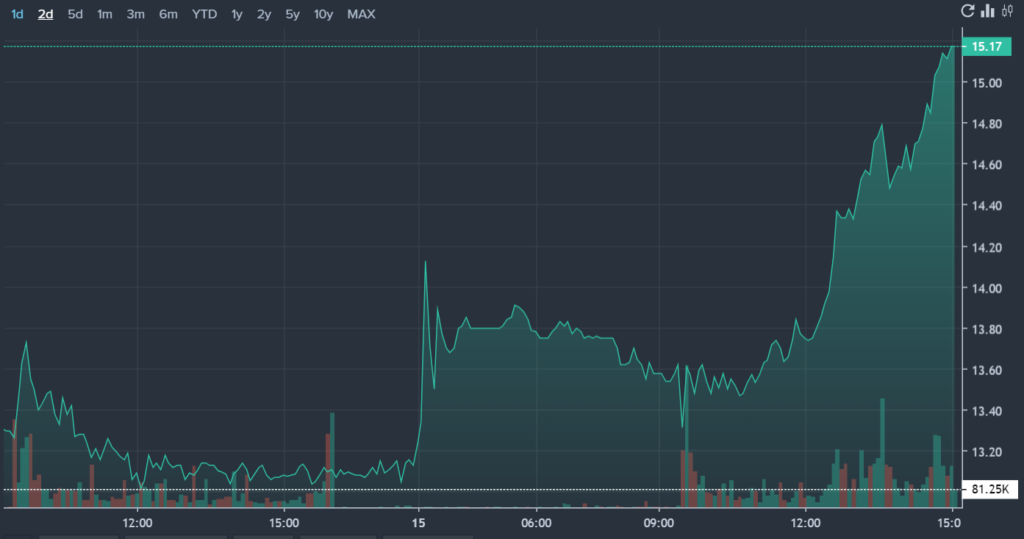
Additionally, Novavax has reported an increase in short interest, with 34.36% of the float sold short. Novavax shares were trading with a session volume of 9.306 million compared to the 100-day average volume of 16.057 million. Over the past 52 weeks, the stock has ranged from $3.53 to $23.86.
NVAX Price Action: Novavax shares were up by 15.6% at $15.17 according to Benzinga pro.
See Also:
Photo via Shutterstock.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.