Liquidia Corporation LQDA, on Monday, announced that the Food and Drug Administration (FDA) granted tentative approval of YUTREPIA.
YUTREPIA (treprostinil) inhalation powder received tentative approval for use in treating adults with pulmonary arterial hypertension and pulmonary hypertension related to interstitial lung disease. YUTREPIA could gain final approval from the FDA once Tyvaso DPI's regulatory exclusivity expires in May 2025.
"We are disappointed and disagree with the FDA's decision to simultaneously grant regulatory exclusivity to United Therapeutics for Tyvaso DPI that encompasses chronic use of essentially any dry-powder formulation of treprostinil in the approved indications for a three-year period for its new dosage form approved on May 23, 2022," said Roger Jeffs, CEO of Liquidia.
Liquidia shares fell 30.6% to close at $9.79 on Monday.
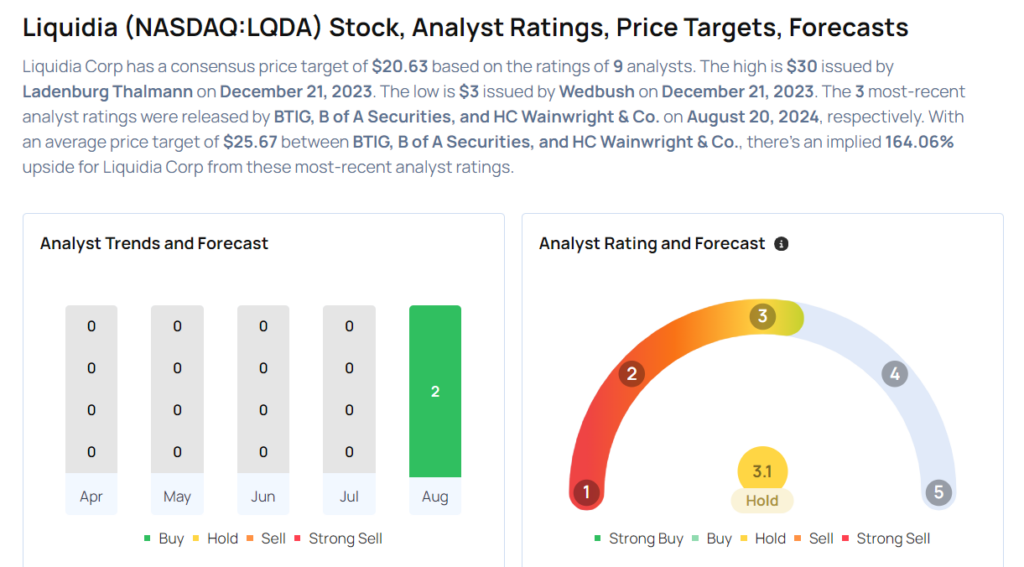
These analysts made changes to their price targets on Liquidia following earnings announcement.
- BTIG analyst Julian Harrison maintained Liquidia with a Buy and lowered the price target from $29 to $25.
- B of A Securities analyst Greg Harrison maintained the stock with a Buy rating, while cutting the price target from $24 to $23.
- HC Wainwright & Co. analyst Andrew Fein maintained Liquidia with a Buy and slashed the price target from $32 to $29.
Considering buying LQDA stock? Here’s what analysts think:
Read Next:
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
date | ticker | name | Price Target | Upside/Downside | Recommendation | Firm |
|---|
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.