Zinger Key Points
- Novavax's stock is moving higher following FDA approval of its 2024-2025 COVID-19 vaccine
- Short interest has dropped 17.52%, signaling reduced bearish sentiment, which may further contribute to stock volatility.
- Find out which stock just claimed the top spot in the new Benzinga Rankings. Updated daily— discover the market’s highest-rated stocks now.
Novavax Inc. NVAX shares are on watch Thursday. Here’s a recap of recent events.
Key Developments:
- Short Interest: Novavax's short interest has fallen, signaling that fewer investors are betting against the stock. It would take about 3.09 days to cover all short positions based on average trading volumes.
- FDA Approval: Novavax's updated COVID-19 vaccine has been approved and will be available at thousands of locations across the U.S., including retail pharmacies and grocers. This approval strengthens Novavax's position in the market as it continues to offer a unique protein-based vaccine alternative.
- Revenue Guidance: In August, Novavax lowered its 2024 revenue guidance to between $700 million and $800 million, significantly below earlier projections.
In comparison, Moderna Inc. MRNA also shared updates during its R&D day, projecting 2025 revenues of $2.5 billion to $3.5 billion, below the consensus of $3.74 billion. The company is cutting its R&D budget by $1.1 billion by 2027 and focusing on ten prioritized products. Despite lower-than-expected revenue forecasts, Moderna remains confident in its long-term growth and cash reserves.
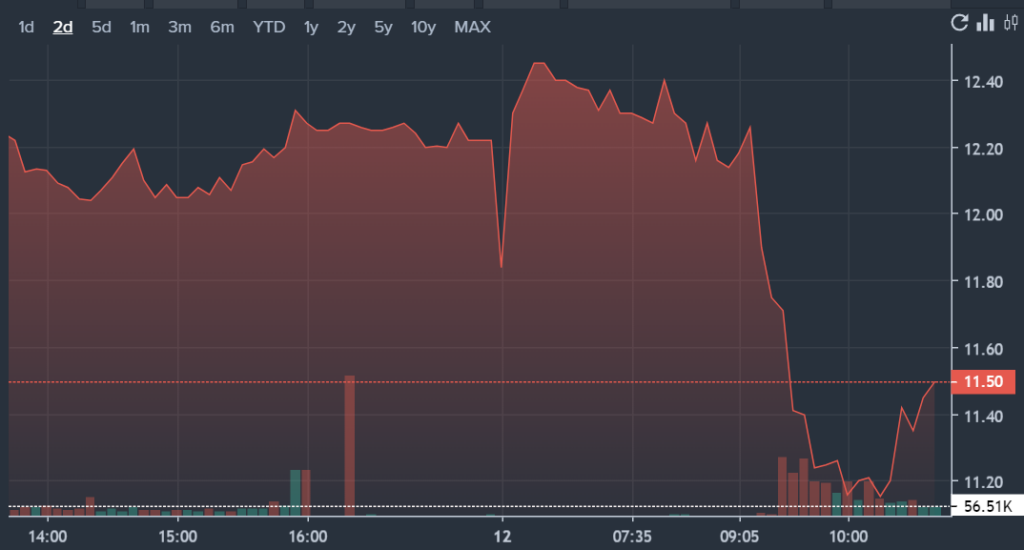
NVAX Price Action: Novavax shares were down by 2.48% at $11.99 according to Benzinga Pro.
See Also:
Photo via Shutterstock.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.