Shares of Ionis Pharmaceuticals IONS and Biogen BIIB lost at least 2% in market cap on Thursday after the companies announced their decision to terminate the development of their investigational amyotrophic lateral sclerosis (ALS) drug, BIIB105 / ION541.
This decision was based on topline data from the phase I/II ALSpire study, wherein treatment with the drug did not slow disease progression. This was evident from the six-month data from the study wherein ALS patients treated with BIIB105 did not achieve a reduction in levels of plasma neurofilament light chain (NfL), a marker of neurodegeneration and neuronal damage.
Patients treated with BIIB105 also failed to demonstrate an impact on clinical outcome measures of function, breathing and strength.
However, this wasn't the only partnership with Ionis that Biogen walked away from. In a separate press release, the pharma giant announced its decision to opt out of licensing the rights to develop BIIB121 / ION582, an experimental Angelman Syndrome drug. However, Biogen did not specify any rationale for this decision.
Year to date, shares of Biogen are down 11.1% compared to the industry‘s 4.6% fall.
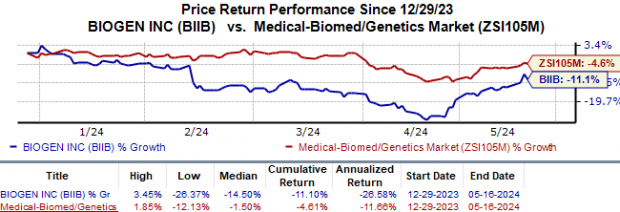
Image Source: Zacks Investment Research
Despite Biogen's departure, Ionis intends to advance ION582 independently as part of its wholly-owned pipeline. In fact, it also posted positive topline results from a phase I/IIa study (called HALOS) evaluating the Angelman Syndrome drug.
A rare genetic disorder, Angelman Syndrome causes developmental disabilities and nerve-related symptoms. Currently, there are no approved treatments for Angelman Syndrome.
Though Ionis did not report any numbers, it did state that treatment with ION582 was also safe and well tolerated in study participants. In fact, patients treated with ION582 achieved consistent improvements across multiple functional domains, including cognition, communication and motor function. Detailed data from this study will be presented at the upcoming Angelman Syndrome Foundation meeting.
Based on the above results, Ionis intends to advance the candidate to a pivotal study. To finalize the study design, it intends to review the results with regulatory authorities.
Ionis' shares have lost 25.6% in the year so far compared with the industry's 5.8% fall.
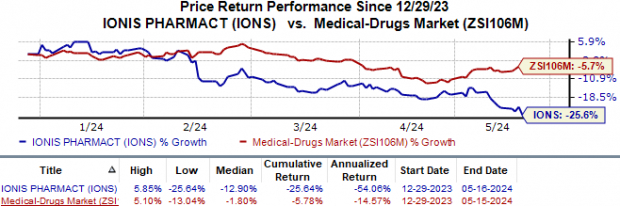
Image Source: Zacks Investment Research
Though Biogen and Ionis are not working on the ALS and Angelman Syndrome programs together, the two companies harbor a long-standing partnership that has been useful in other areas. The first fruit born out of this collaboration was Spinraza, a blockbuster medication approved for treating spinal muscular atrophy.
Despite the ALSpire study failure, Biogen and Ionis have achieved success in the ALS space. Post Spinraza, Qalsody is the second marketed drug born out of this partnership. The drug was approved by the FDA last year for treating ALS with superoxide dismutase 1 (SOD1) mutations.
Apart from the above areas, Biogen and Ionis are developing numerous investigational medicines to treat neurodegenerative diseases like Alzheimer's disease and Parkinson's disease.
Zacks Rank & Key Picks
Ionis and Biogen carry a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector include Ligand Pharmaceuticals LGND and Heron Therapeutics HRTX, each carrying a Zacks Rank #2 (Buy) at present.
In the past 60 days, estimates for Ligand Pharmaceuticals' 2024 earnings per share have risen from $4.42 to $4.56. During the same period, EPS estimates for 2025 have improved from $5.11 to $5.27. Year to date, LGND's shares have appreciated 19.0%.
Earnings of Ligand Pharmaceuticals beat estimates in each of the last four quarters. Ligand delivered a four-quarter average earnings surprise of 56.02%.
In the past 60 days, estimates for Heron Therapeutics' 2024 loss per sharehave improved from 22 cents to 14 cents. During the same period, loss estimates for 2025 have narrowed from 9 cents to 2 cents. Year to date, shares of HRTX have surged 89.4%.
Earnings of Heron Therapeutics beat estimates in three of the last four quarters while missing the mark on one occasion. HRTX delivered a four-quarter average earnings surprise of 30.33%.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.

