DexCom Inc. DXCM and Abbott Laboratories ABT are likely to launch their first over-the-counter (OTC) continuous glucose monitors (CGMs) in the coming months of 2024.
Dexcom's Stelo and Abbott's Lingo & Libre Rio received FDA clearance in March and June this year, respectively, and now they are up for launch in the coming months of 2024. These devices use the same hardware as past CGMs but are intended for people who do not take insulin.
The new devices are likely to allow the companies to reach about 25 million people in the United States with Type 2 diabetes who do not take insulin, 15 million people who have been diagnosed with pre-diabetes and an estimated 85 million people who have undiagnosed pre-diabetes. This represents a significant market opportunity for both companies.
More on the Launch
With Stelo CGM, Dexcom is likely to target people with Type 2 diabetes who do not take insulin. However, Stelo can also be used by non-diabetic people, thanks to a broad label from the FDA. Abbott, on the other hand, will split its OTC CGM into two sensors: Lingo, which is intended for people who do not have diabetes, and Libre Rio, which will compete more directly with Dexcom for Type 2, non-insulin users.
Abbott is offering two different OTC CGMs as the company believes that "there's no one size fits all approach to managing care." Per the company, people living with diabetes might want features for insulin management, medication tracking and sharing data with a provider or caregiver. However, Dexcom's Stelo is the only OTC CGM that is intended for people with Type 2 diabetes. The company expects that a large number of non-diabetic people will try the device.
Dexcom is likely to plan a late August launch of its Stelo sensor. The company is looking forward to first selling the device online, which can prove beneficial for the company to learn more about users and their buying patterns. Abbott also intends to launch an e-commerce website this summer to offer its wellness-focused Lingo device. The company has not yet revealed any schedule for Libre Rio.
On the pricing front, both companies have not disclosed pricing for the upcoming products. Dexcom's Stelo is likely to be competitive with other cash-pay products. In the United Kingdom, where Abbott first launched its Lingo device, the company charges roughly around $150 a month.
More on Dexcom's Stelo
Dexcom announced the FDA clearance of its Stelo OTC glucose biosensor to consumers without a prescription in March 2024. The G7, the company's most recent CGM sensor, is presently prescribed. With Stelo's approval for non-prescription use, people without insurance coverage for CGM would have even easier access to state-of-the-art CGM technology.
The development of Stelo, which was made possible by the Dexcom G7 makers, took into consideration the special requirements of adults with Type 2 diabetes who are over 18 and do not need insulin. The gadget is a tiny wearable sensor that is placed on the back of the upper arm and sends data on blood sugar levels straight to a user's smartphone.
Stelo's software is designed to onboard people who have never used a CGM, as well as to educate them about what blood glucose means and how changes throughout the day are normal. The product's main purpose is to help people manage their diet and activities to lower their average glucose.
More on Abbott's OTC CGMs
Abbott announced the FDA clearance of its Lingo & Libre Rio CGMs, which are based on Abbott's world-leading FreeStyle Libre CGM technology, in June 2024. The newly cleared systems have been intentionally designed to meet different needs: Lingo for consumers who want to better understand and improve their health and wellness, and Libre Rio for adults with Type 2 diabetes who do not use insulin and typically manage their diabetes through lifestyle modifications.
Abbott's consumer bio-wearable, Lingo, is designed for consumers 18 years and older who are looking to improve their overall health and wellness. Lingo is set to track glucose and provide personalized insights and customized coaching to help people create healthy habits, retrain their metabolism and improve their overall well-being. The Lingo system combines a biosensor that is worn on the upper arm for 14 days and continuously streams glucose data to a coaching application on a smartphone, translating the body's language and giving insights on the person's reaction to food, exercise and life's daily stressors.
Libre Rio is Abbott's first OTC CGM system for people with diabetes in the United States. It is designed for people aged 18 and older with Type 2 diabetes who do not use insulin and typically manage their diabetes through lifestyle modifications. Libre Rio is the first OTC CGM system with a measurement range of 40-400 mg/dL, allowing for the measurement of extremely low or high glucose events.
Expected Revenue Growth
Dexcom has estimated that Stelo is likely to bring $40 million in sales this financial year. Even though Abbott has refused to provide any sales estimates for its upcoming devices, the company anticipates its diabetes business to reach $10 billion in revenues by 2028. A better commercialization plan and a strong sales team should help the companies gain market share in this new segment.
Abbott has significantly higher resources with an extensive presence in healthcare product markets. This is expected to help the company gain an advantage over DexCom as commercialization of Stelo and Lingo begins this year. However, DexCom has significant success with its CGM devices in the United States, and a similar trend may follow in the OTC glucose sensor market amid competition from a larger device maker. The companies will likely provide updates on their OTC glucose sensors during their next earnings call.
Industry Prospects
Per a report by MarketsandMarkets, the global digital diabetes management market was estimated to be $18.9 billion in 2023 and is expected to reach $35.8 billion by 2028 at a growth rate of 13.6%.
The market is being driven by escalating diabetes care solutions and technological developments that have made it possible to introduce highly adaptable solutions. Other significant drivers include the increasing popularity of the use of connected devices and apps as well as the growing adoption of cloud-based solutions.
Price Performance
In the past six months, DXCM's shares have lost 10.5%, and ABT's shares have plunged 7.6% against the medical sector's 2.4% growth. The S&P 500 has increased 18.7% in the same time frame.
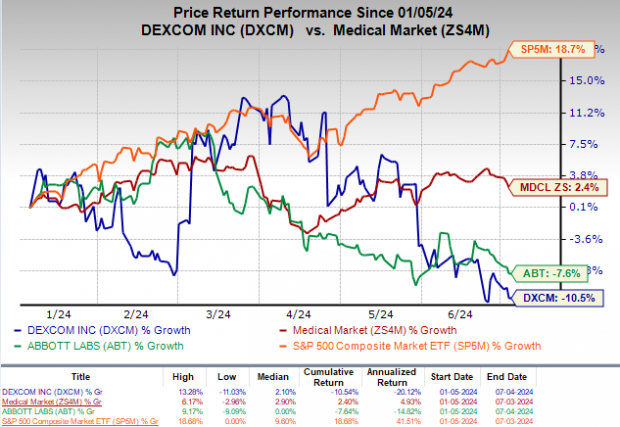
Image Source: Zacks Investment Research
Zacks Rank & Other Stocks to Consider
DXCM presently carries a Zacks Rank #2 (Buy).
A couple of other top-ranked stocks in the broader medical space that have announced quarterly results are DaVita DVA and Ecolab ECL.
DaVita, carrying a Zacks Rank of 2 at present, has an estimated long-term growth rate of 13.6%. DVA's earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 29.4%.
DaVita's shares have gained 44% compared with the industry's 20.4% growth in the past year.
Ecolab, carrying a Zacks Rank of 2 at present, has an estimated long-term growth rate of 13.3%. ECL's earnings surpassed estimates in each of the trailing four quarters, the average surprise being 1.7%.
Ecolab's shares have gained 33.8% against the industry's 9.3% decline in the past year.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.