Zinger Key Points
- Tivic Health shares surge over 100% after the company reports positive meetings with White House and FDA officials.
- The company says it discussed expedited approval paths for its radiation treatment drug Entolimod.
- Today's manic market swings are creating the perfect setup for Matt’s next volatility trade. Get his next trade alert for free, right here.
Shares of Tivic Health Systems, Inc. TIVC are soaring Tuesday after the company announced it held senior-level briefings with White House officials and the U.S. Food and Drug Administration (FDA), which resulted in strong interest in potential military and defense product candidates.
What To Know: Tivic disclosed that it presented its therapeutic programs to senior leadership at the White House and FDA on Thursday. Discussions centered around the company's TLR5 and non-invasive cervical vagus nerve stimulation (ncVNS) programs.
The White House meeting focused on the TLR5 program and its lead product candidate, Entolimod, which is being developed to treat acute radiation syndrome. The briefing addressed the need for better treatment options for radiation exposure and highlighted Entolimod's role in enhancing immune response through the TLR5 pathway, which is part of the body's innate immune system.
The company noted that Entolimod previously received significant development funding from various U.S. government agencies, including BARDA, NASA, the Defense Threat Reduction Agency, the Department of the Army and the Department of Defense. Tivic emphasized the potential for Entolimod to offer advantages over current treatments, such as providing gastrointestinal and hematopoietic protection from radiation in a single dose.
In a separate meeting, Tivic met with senior leadership at the FDA to discuss potential expedited regulatory pathways for both Entolimod and Entolasta, a second-generation TLR5 agonist.
The company also presented its ncVNS program to the FDA, which is targeting a range of inflammatory, cardiac and neurologic conditions. The FDA gave favorable feedback, including potential support for breakthrough device designation upon completion of relevant clinical work.
“We deeply appreciated the opportunity to discuss the importance of our clinical pipeline in potentially protecting and healing both active and retired military personnel, first responders, as well as U.S. citizens and our allies,” said Jennifer Ernst, CEO of Tivic Health.
“While these meetings were informal in nature, we expect to continue these discussions as Entolimod and our ncVNS medical programs progress toward potential FDA approval.”
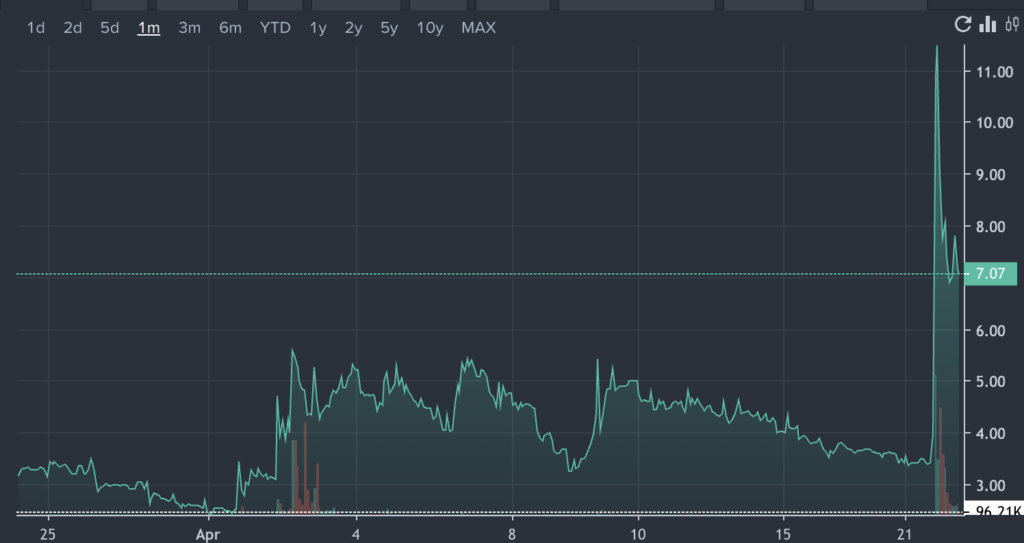
TIVC Price Action: Tivic Health shares traded as high as $13.23 on Tuesday. The stock was up 107.7% at $7.28 at the time of publication, according to Benzinga Pro.
Read Next:
Image via Shutterstock.
Edge Rankings
Price Trend
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.