By Bill Griffin
The emerging European cannabidiol (CBD) market has been thrown into disarray since January 2019 over a change to CBD's classification by the European Food Safety Authority (EFSA).
Here we look at what changed and explore options for those companies impacted.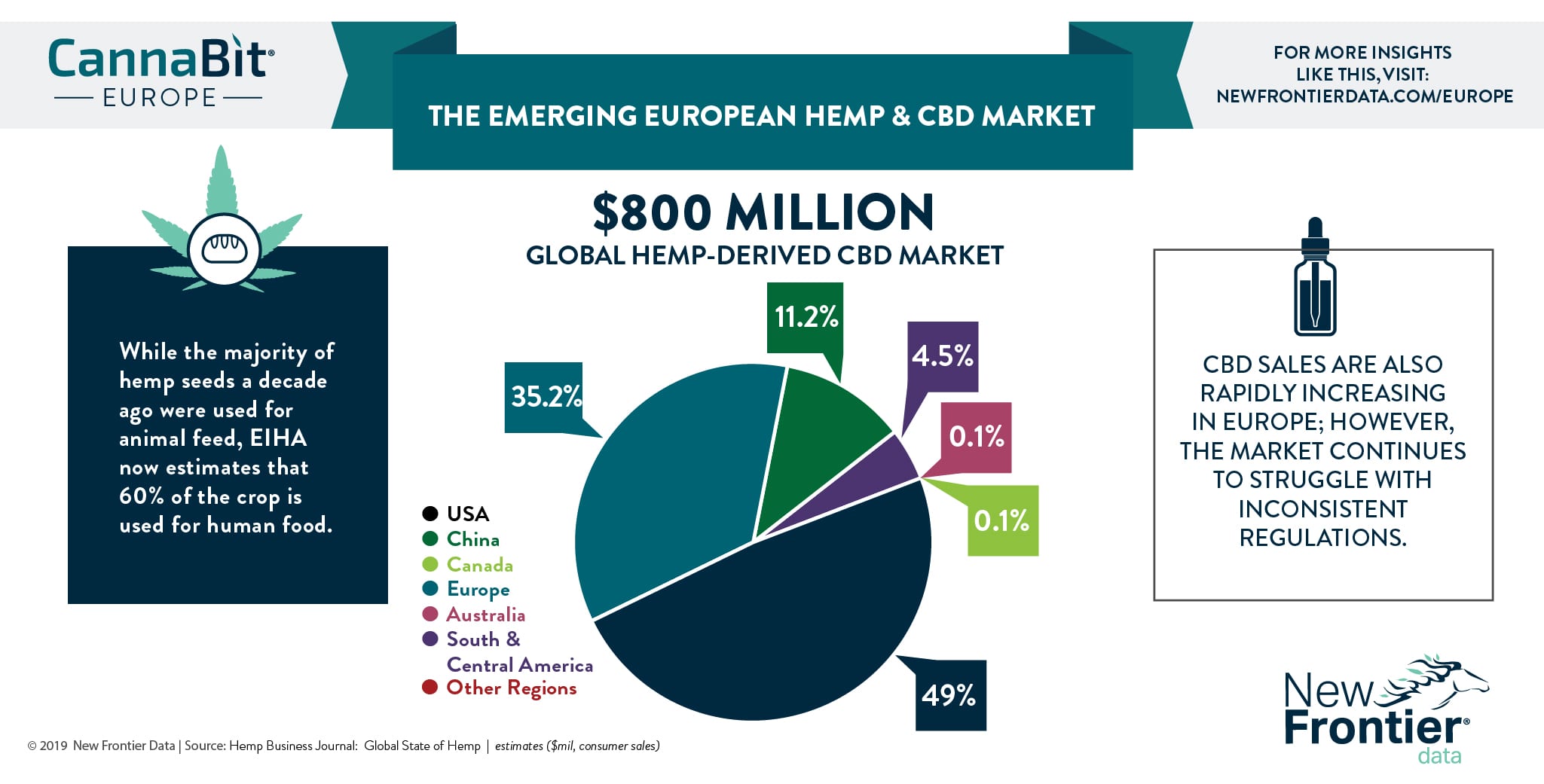
What changed?
Before January 2019, the entry for CBD in EFSA's novel foods catalogue read:
Cannabidiol
Extracts of Cannabis sativa L in which cannabidiol (CBD) levels are higher than CBD levels in the source Cannabis sativa L are novel in food. Cannabidiol (CBD) is one of the cannabinoids of the Cannabis sativa plant. In the European Union, cultivation of Cannabis sativa L. varieties is granted provided that they are registered in the EU's "Common Catalogue of Varieties of Agricultural Plant Species", and the tetrahydrocannabinol (THC) content does not exceed 0.2 % of the plant.
On 17 January, a new entry was added for generic "cannabinoids", while the entry for CBD links to a new entry stating "products containing cannabinoids are considered novel foods as a history of consumption has not been demonstrated."
As a result, CBD went from being permitted for food in Europe so long as its THC content was below 0.2%, to being considered a fully unauthorised novel food. Member states are thus left to decide on the legality of CBD, and some are blocking sales based on the classification.
What is the EFSA?
The EFSA is a governmental agency funded by the European Union while operating independently of the European Commission, Council, Parliament, and EU Member States. It was established by the EU in 2002 as a source of scientific advice regarding risks associated within the food chain.
The EFSA covers a wide spectrum of foodstuffs and additives. Novel food represents one category among many including food supplements, nutrition, and animal feed. The EFSA is responsible for scientific considerations about foods, not policy.
What is "Novel Food"?
According to the European Commission, novel food is defined as food "that had not been consumed to a significant degree by humans in the EU before 15 May 1997, when the first Regulation on novel food came into force."
An example of an authorised novel food is the annual herb nigella sativa; its seeds are commonly known as black cumin, and have historically been used as a spice in the Balkans.
Being listed in the novel food catalogue is not inherently bad. The EFSA says that "any food business operator can place an authorised Novel Food on the European Union market.". However, as it stands, CBD is classed as an "unauthorised Novel Food".
CBD-rich hemp has been eaten in Europe for millennia.
The European Industrial Hemp Association (EIHA) argues that CBD is a food supplement – as per many plant-derived substances such as valerian and ginkgo biloba – so should not be classified as novel food.
When pushed by member states for clarification on the status of CBD, the EFSA concluded that it was an extraction of cannabis sativa, and therefore has not been used in foodstuffs pre-15 May 1997. The agency added that CBD is present in hemp-derived food, and thus been used in foodstuffs pre-1997.
As the EIHA clarified, "CBD is a natural constituent in hemp food which has been used in Europe for 2,000 years. The European Commission stated on 18 December 1997 that food containing parts of the hemp crop is not considered ‘novel food'."
EIHA President Mark Reinders explained that "all parts of the industrial hemp plant are food, so the extracts from these parts – as long as traditional extraction technology is being used – can't be considered novel food. Whole-plant extracts are not novel food in this wording."
Given that, it seems permissible that a company may bring a CBD-rich product to market by using a "traditional" extraction technology.
Can CBD's status change?
The ICCI (International Cannabis & Cannabinoids Institute) recommends that European producers of CBD and hemp extracts apply for novel food authorization.
ICCI's Martin Másílko advises that "companies need to apply directly to their member state authority who will in turn put in an application to EFSA. ICCI are currently helping five European companies make these applications."
The process for consulting a Member State to clarify novel food status is governed by the Commission Implementing Regulation (EU) 2018/456.
Currently, there is only one application being processed. A verdict was supposed to be given by the end of March 2019, though as of July there has been no clarification. Should the decision be positive it could allow for a daily intake of 130mg of CBD for adults.
The post EFSA's CBD Novel Food Classification appeared first on New Frontier Data.
Image Sourced by Pixabay
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
Cannabis is evolving – don’t get left behind!
Curious about what’s next for the industry and how to leverage California’s unique market?
Join top executives, policymakers, and investors at the Benzinga Cannabis Market Spotlight in Anaheim, CA, at the House of Blues on November 12. Dive deep into the latest strategies, investment trends, and brand insights that are shaping the future of cannabis!
Get your tickets now to secure your spot and avoid last-minute price hikes.