Denali Therapeutics Inc. DNLI is having a good run in 2024. Shares of this biopharmaceutical company have risen 11.9% in the past three months against the industry's decline of 6%.
Denali is developing a broad portfolio of product candidates engineered to cross the blood-brain barrier (BBB) for the treatment of neurodegenerative and lysosomal storage diseases. The recent upward price trajectory can be attributed to positive updates.
Earlier in the month, the company announced that the FDA has selected its experimental candidate, DNL126, in support of clinical trials advancing the rare disease therapeutics (START) Pilot Program. The candidate is an investigational enzyme replacement therapy designed to cross the BBB for the potential treatment of MPS IIIA (Sanfilippo syndrome type A).
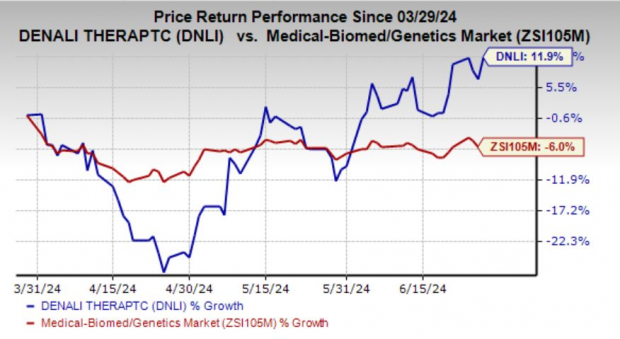
Image Source: Zacks Investment Research
The newly launched START Pilot program was initiated to accelerate the pace of the development of novel drugs that are intended to address the significant need in the treatment of rare diseases.
Denali is conducting a multicenter, open-label, phase I/II study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics and exploratory clinical efficacy of DNL126 in participants with MPS IIIA. Since DNL126 has been selected as a START participant, it can interact with the FDA frequently. The company expects the increased level of engagement from the FDA to facilitate alignment on the most efficient development path to ultimately support a marketing application for DNL126 in MPS IIIA.
The pipeline progress has been encouraging. DNLI has collaborations with bigwigs like Biogen BIIB and Sanofi SNY for some of its candidates.
Denali and Biogen are jointly evaluating a leucine-rich repeat kinase 2 inhibitor (LRRK2), BIIB122/DNL151, which is in development for the treatment of Parkinson's disease (PD).
In February, Denali entered into a funding agreement with a third party related to a global phase IIa study of BIIB122/DNL151. The company plans to solely operationalize the study to evaluate the safety and biomarkers associated with BIIB122 in participants with PD and confirm pathogenic variants of LRRK2.
This agreement includes a committed funding of $75.0 million, of which $12.5 million was received in January 2024, and the remainder will be triggered based on time and operational milestones in the study. Denali plans to initiate the phase IIa study in 2024. Denali will pay low single-digit royalties to the third party on annual worldwide net sales of LRRK2 inhibitors for the treatment of PD, with royalty amounts varying based on the scope of the label.
Biogen continues to conduct the ongoing global phase IIb LUMA study in early-stage PD patients. Denali and Biogen will co-commercialize the candidate upon regulatory approval.
Please note that Denali has another candidate in its pipeline, tividenofusp alfa (DNL310), which is engineered to cross the BBB and restore duronate 2-sulfatase and reduce glycosaminoglycans, both peripherally and in the brain, in individuals with mucopolysaccharidosis II (MPS II or Hunter syndrome).
In February, new positive data from the ongoing phase I/II study of tividenofusp alfa in MPS II were presented. Enrollment in the phase II/III COMPASS study on the candidate is expected to be completed this year.
Denali believes that the Center for Drug Evaluation and Research (CDER) division of the FDA may be open to discussing an accelerated path for tividenofusp alfa, based on continued dialogue.
Meanwhile, Denali and partner Sanofi are co-developing SAR443820/DNL788. The ALS study on this candidate has been discontinued based on the results of the phase II HIMALAYA study, which did not meet the primary endpoint. Nonetheless, Sanofi continues to evaluate SAR443820 in another phase II study for the treatment of multiple sclerosis.
Denali and Sanofi are also developing SAR443122/DNL758 (eclitasertib), a peripheralRIPK1 inhibitor, for the treatment of ulcerative colitis.
The cash position is sound as well. Cash, cash equivalents and marketable securities totaled approximately $1.43 billion as of Mar 31, 2024. In February, Denali announced the completion of a private investment in public equity (PIPE) financing with gross proceeds of $500 million.
Denali's pipeline potential and the successful development of any of these candidates will be a significant boost to the company. These should also enable DNLI to maintain its upward trajectory. However, any pipeline setback will be a major blow for the company.
Zacks Rank and a Stock to Consider
Denali currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the biotech sector is ALX Oncology Holdings ALXO, which currently carries a Zacks Rank #2 (Buy).
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology's 2024 loss per share has remained constant at $2.89. During the same period, the consensus estimate for 2025 loss per share has remained constant at $2.73. ALX Oncology's earnings beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.